Attention iOS users: You will not be able to view tables on this page without Adobe Flash Player, which is not supported by iOS. You can click on links to tables, or install a web browser with Flash support, such as Puffin.
Desktop users: You cannot print from the Scribd table format. For a printed copy, click on the link to the table and print from your browser.
Android users: The Puffin browser is also recommended for android phones, such as the Samsung Galaxy S4. If you are interested in the analytical data in the tables, this page is best viewed with your desktop.
Desktop users: You cannot print from the Scribd table format. For a printed copy, click on the link to the table and print from your browser.
Android users: The Puffin browser is also recommended for android phones, such as the Samsung Galaxy S4. If you are interested in the analytical data in the tables, this page is best viewed with your desktop.
Equine Nutrition (Stephanie Ohlemacher and George Lager)
Stephanie Ohlemacher is a Natural Hoof Care Practitioner/Clinician and practicing Registered Nurse with ICU/ER and OR surgery experience. She has studied hoof care under a number of prominent barefoot instructors, but ultimately her best teacher is the horse. She is a strong proponent of natural holistic trimming, utilizing natural horsemanship and alternative therapies. Ms. Ohlemacher has extensive experience (20 years) with a wide variety of equines, including pleasure horses, miniatures to drafts, gaited horses, foals and mares, stallions, mustangs and donkeys. She uses conventional tools as well as abrasive trimming (angle grinder/flap disc), and offers basic and abrasive hoof care clinics at her farm near Elizabeth, Indiana. If you are unfamiliar with abrasive trimming, link to her video on YouTube. E-mail: sonaturalhoofcare@gmail.com.
Stephanie with Stormy, one of 15 horses at her Spring Lake Farm, near Elizabeth, Indiana.
This page will present some basic and easily implemented ideas on nutrition that will keep your horse, mule, or donkey happy and healthy. A number of equine health issues are related to nutritional imbalances. For example, ongoing equine studies show that balancing the diet can significantly benefit those equines diagnosed with acute and chronic laminitis (sometimes referred to as founder), insulin resistance (IR), equine Cushing's disease/syndrome and obesity. For a more information, refer to Dr. Eleanor Kellon's web site on nutrition.
There are currently seven parts to this web page. You can work your way through the entire page, or link separately to each part.
Part 1. Carbohydrate levels in hay and grass forage
Part 2. Balancing equine diets
Part 3. Choosing and formulating your own supplements
Part 4. Selenium: NRC requirements and geochemistry
Part 5. Biochemical function of deficient minerals in Harrison County, Indiana hay/pasture
Part 6. Iodine deficiency and supplement analysis
Part 7. Interpreting well water analyses in Indiana karst (limestone) terranes
Part 1. Carbohydrate levels in hay and grass forage
Part 1 was originally posted in May 2013 as an laminitis alert for equine owners in Harrison and adjoining counties in southern Indiana and Kentucky. Based on input from veterinarians and hoof care providers, there was a higher than normal incidence of acute laminitis cases last spring. It's that time of year again so take precautions for your at-risk horses. If this an emergency situation, go directly to Take-home message at the end of this page for further information.
Now that the cold weather has returned, you might want to read this article by Kellon (2014) on winter laminitis, particularly if your horse has had recurrent bouts of laminitis.
Now that the cold weather has returned, you might want to read this article by Kellon (2014) on winter laminitis, particularly if your horse has had recurrent bouts of laminitis.
Harrison County, Indiana lies just west of the Louisville Metro area. The county's southern half borders Kentucky along the Ohio River.
If your equine is at risk, high-carbohydrate (sugar) levels in grasses may trigger hoof soreness or laminitis, as diagnosed by a veterinarian. In most cases, the ideal time to graze IR equines, or those with chronic laminitis, is late night to mid-morning when carbohydrates are lower. The rate of carbohydrate production from photosynthesis during sunny days is greater than the rate of consumption from plant respiration, resulting in peak concentrations of carbohydrates in the afternoon (Fig. 1).
During warm, cloudy days, the rate of photosynthesis will be reduced and more of the stored carbohydrates will be consumed by respiration. After several successive cloudy days, sugar content may be low enough to graze at-risk horses in the afternoon.
During warm, cloudy days, the rate of photosynthesis will be reduced and more of the stored carbohydrates will be consumed by respiration. After several successive cloudy days, sugar content may be low enough to graze at-risk horses in the afternoon.
Figure 1. Stylized graph showing the relationship between carbohydrate storage (photosynthesis) and consumption (respiration) during a typical cloudless day in spring. The net gain in carbohydrates during the day is represented by the light green shaded area at the top of the bell-shaped curve. During the night carbohydrates are used for plant growth. At the compensation points, rates of photosynthesis and respiration are equal, i.e., carbohydrates produced are equal to those consumed. Carbohydrate-intolerant equines should not be grazed during "NO GRAZING" times when carbohydrate levels are highest (approximately 10:00 AM to 11:00 PM).
A comprehensive treatment of carbohydrates in forage is given by Kathryn Watts at safergrass.org. Included on the safergrass web site is an article on "Pasture Management to Minimize the Risk of Equine Laminitis".
We are currently building a database of carbohydrate levels in grasses and hays in Harrison County, Indiana, in response to different environmental conditions (e.g., time of day, grass maturity, drought, overgrazing, sunny versus cloudy skies, dramatic changes in weather conditions, and soil nutrient levels). Some preliminary results are presented Table 1 below. Samples were collected based on the procedures recommended by Equi-analytical Laboratory ("Taking a Sample").
Pasture samples were placed in a conventional freezer at -17 C (0 F) within 30 minutes and shipped overnight with ice packs in insulated containers. Pelletier et al. (2010) have compared carbohydrate concentrations in forage samples using different drying procedures. Water soluble carbohydrates (WSC% in Table 1) determined after freezing spring growth (timothy) at -20 C (- 4 F) for 1 month followed by drying at 55 C (113 F) for 48 hours are ~10% lower than those obtained after freeze drying. This difference is comparable to errors associated with sampling and some nutrient analyses.
We are currently building a database of carbohydrate levels in grasses and hays in Harrison County, Indiana, in response to different environmental conditions (e.g., time of day, grass maturity, drought, overgrazing, sunny versus cloudy skies, dramatic changes in weather conditions, and soil nutrient levels). Some preliminary results are presented Table 1 below. Samples were collected based on the procedures recommended by Equi-analytical Laboratory ("Taking a Sample").
Pasture samples were placed in a conventional freezer at -17 C (0 F) within 30 minutes and shipped overnight with ice packs in insulated containers. Pelletier et al. (2010) have compared carbohydrate concentrations in forage samples using different drying procedures. Water soluble carbohydrates (WSC% in Table 1) determined after freezing spring growth (timothy) at -20 C (- 4 F) for 1 month followed by drying at 55 C (113 F) for 48 hours are ~10% lower than those obtained after freeze drying. This difference is comparable to errors associated with sampling and some nutrient analyses.
Your browser does not support viewing this document. Click here to download the document.
Pasture samples
A cold front passed through the area two days before sample collection resulting in warm, sunny days and freezing nights (Fig. 2).
A cold front passed through the area two days before sample collection resulting in warm, sunny days and freezing nights (Fig. 2).
Figure 2. Variation of maximum, mean and minimum temperature for the period May 10, 2013 to May 16, 2013 for the weather station at Brandenburg, Kentucky. Minimum temperatures at sample locations in southern Indiana near Central and Elizabeth were slightly less than 32 F with light frost. Red vertical line indicates sampling date. Image from archived weather history at weathersource.com.
Total sugar content is defined as ESC% + Starch% (dm) (Table 1, highlighted in red). A value less than ~10% is generally recommended for obese, laminitic, or IR equines. In a number of studies, WSC% + Starch% is used as an upper limit for sugar content of hays fed to laminitic equines; however, the fructan component of WSC% is probably not a high-risk factor in most pasture laminitic cases, particularly in the spring (more on this later). Fructan is not a sugar but a complex carbohydrate consisting of chains of fructose molecules.
"Pasture 1" was collected at 8:00 AM near Central, Indiana with the presence of a light frost (Fig. 3). Table 1 shows that total sugars are not excessively high, even though the sample was collected under environmental conditions that can cause "cold stress", i.e., decrease in plant respiration and increase in carbohydrate storage. Based on the carbohydrate level only, this pasture would be acceptable for grazing at-risk equines in the early morning. However, to avoid overeating and consuming too much carbohydrate, more limited access to this pasture may be required. The high fiber content NDF% of the mature grass is consistent with its lower carbohydrate content WSC% (dm) (Fig. 1, Watts 2008).
"Pasture 2" was collected at 5:30 PM on the same day from an overgrazed pasture near Elizabeth, Indiana (Fig. 3). This sample has twice the sugar content and is less fibrous (Table 1). The grass is short, which causes carbohydrates to concentrate at the base of the grass stems. Although the intake may be less, equines at risk should be removed from this pasture during the afternoon and provided low-sugar hay in a grass-free area, preferably a dry lot, or Paddock Paradise, rather than a stall.
"Pasture 2" was collected at 5:30 PM on the same day from an overgrazed pasture near Elizabeth, Indiana (Fig. 3). This sample has twice the sugar content and is less fibrous (Table 1). The grass is short, which causes carbohydrates to concentrate at the base of the grass stems. Although the intake may be less, equines at risk should be removed from this pasture during the afternoon and provided low-sugar hay in a grass-free area, preferably a dry lot, or Paddock Paradise, rather than a stall.
Figure 3. Photos of Pasture 1 (left) and Pasture 2 (see Table 1) taken about 2 weeks after sampling. The overgrazed pasture with short grass has twice the sugar content as the mature, ungrazed pasture. Note that seed heads in mature pastures are high in carbohydrates (starch) and should not be consumed by at-risk equines. Remove seed heads by mowing, or by grazing pastures with ruminants, or equines not at risk.
Instead of restricting turnout, consider a grazing muzzle (Fig. 4) during high carbohydrate times. Daily movement is NECESSARY for symptom reversal of at-risk equines. Feed low-carbohydrate hay prior to placing grazing muzzle.
Figure 4. "Blue" suffered an acute laminitis attack during grazing high-sugar grass in overgrazed Pasture 2 (Fig 3). She was immediately removed from pasture, given phenylbutazone (bute) for pain relief, booted, and fed Dr. Kellon's Emergency Diet. The following day when she was showing less discomfort, excess hoof wall length was removed, preserving sole and frog integrity. After trimming, Blue was rebooted and confined to paddock with low-sugar grass hay. Several days later she was fitted with grazing muzzle, and released to a second pasture that was less grazed. Case study to follow with ongoing treatment and dietary adjustments.
Hay samples
Table 1 shows that sugar content in hay samples varies from 4.3 to 9.0%, a carbohydrate content acceptable for equines in good health. Hay 10 with 4.3% sugar contains a significant amount of timothy, a cool season grass noted for its low-sugar content. For more information on Purina Hydration Hay (Hay 11), go to Purinahorsehayblocks.com.
To reduce sugar content for at-risk equines hay can be soaked before feeding. Watts (2003) determined that the average decrease in WSC% (dm) after soaking 15 different hay samples for 30 and 60 minutes (82 F = 28 C) was 19 and 31%, respectively. A more recent study indicates that soaking hay for 20 minutes (46 F = 8 C) will reduce WSC% (dm) by ~5% (In depth:Hay soaking, TheHorse.com). In both studies, sugar loss on soaking was quite variable among hay samples. The greater losses observed by Watts (2003) probably reflect the temperature of the water, i.e., sugars are more soluble in hotter water, and the different harvesting techniques in the U.S. versus U.K., resulting in finer texture and greater surface area of U.S. hays (Longland et al., 2011, Veterinary Record, 06.11.2011). The greater the contact area between water and hay the easier it is to dissolve the sugars. Based on our limited database, most hays in Harrison County, Indiana do not require soaking before feeding.
More specific information on hay composition and texture, and environmental conditions during cutting and baling, will be included for the 2013 hay analyses.
Table 1 shows that sugar content in hay samples varies from 4.3 to 9.0%, a carbohydrate content acceptable for equines in good health. Hay 10 with 4.3% sugar contains a significant amount of timothy, a cool season grass noted for its low-sugar content. For more information on Purina Hydration Hay (Hay 11), go to Purinahorsehayblocks.com.
To reduce sugar content for at-risk equines hay can be soaked before feeding. Watts (2003) determined that the average decrease in WSC% (dm) after soaking 15 different hay samples for 30 and 60 minutes (82 F = 28 C) was 19 and 31%, respectively. A more recent study indicates that soaking hay for 20 minutes (46 F = 8 C) will reduce WSC% (dm) by ~5% (In depth:Hay soaking, TheHorse.com). In both studies, sugar loss on soaking was quite variable among hay samples. The greater losses observed by Watts (2003) probably reflect the temperature of the water, i.e., sugars are more soluble in hotter water, and the different harvesting techniques in the U.S. versus U.K., resulting in finer texture and greater surface area of U.S. hays (Longland et al., 2011, Veterinary Record, 06.11.2011). The greater the contact area between water and hay the easier it is to dissolve the sugars. Based on our limited database, most hays in Harrison County, Indiana do not require soaking before feeding.
More specific information on hay composition and texture, and environmental conditions during cutting and baling, will be included for the 2013 hay analyses.
Comparison of nutrient profiles for alfalfa-rich (Hay 6) and mixed-grass (Hay 7) hays
Table 2 below compares the nutrient profile for a alfalfa-orchard grass hay (Hay 6) and mixed-grass hay (Hay 7) from Central, Indiana. The well-known compositional differences between alfalfa-rich and mixed-grass hays are highlighted in blue. Although alfalfa is relatively low in sugars (Table 1), the higher protein content may not be appropriate for some horses, e.g., adult horses at average activity levels, or those with decreased renal function. Also note the high Ca/P and Ca/Mg ratios which must be balanced to ~2:1, as discussed in the following section.
Your browser does not support viewing this document. Click here to download the document.
Take-home message
1. Reduce sugars/carbohydrates for all equines to below 15%, and
at-risk equines to ~10% or less, including all carbohydrate-laden grains, feeds and treats.
2. Don't overgraze, or mow pastures too short. Both conditions produce plant stress and higher carbohydrate storage.
3. Manage grazing times for at-risk equines by restricting turnout to late evening-early morning hours.
4. Use grazing muzzles for at-risk horses when movement is necessary.
5. Feed low-sugar grass hay. If not tested, soak for ~1 hour.
6. Feed the Emergency Diet for laminitic equines, available through the Equine Cushing's Disease Insulin Resistance Group, Inc.
If you need immediate help, please contact:
Stephanie Ohlemacher,
S.O. Natural Hoof Care
cell: 502-387-7395
home: 812-969-3499
email: sonaturalhoofcare@gmail.com
and consult with your veterinarian.
1. Reduce sugars/carbohydrates for all equines to below 15%, and
at-risk equines to ~10% or less, including all carbohydrate-laden grains, feeds and treats.
2. Don't overgraze, or mow pastures too short. Both conditions produce plant stress and higher carbohydrate storage.
3. Manage grazing times for at-risk equines by restricting turnout to late evening-early morning hours.
4. Use grazing muzzles for at-risk horses when movement is necessary.
5. Feed low-sugar grass hay. If not tested, soak for ~1 hour.
6. Feed the Emergency Diet for laminitic equines, available through the Equine Cushing's Disease Insulin Resistance Group, Inc.
If you need immediate help, please contact:
Stephanie Ohlemacher,
S.O. Natural Hoof Care
cell: 502-387-7395
home: 812-969-3499
email: sonaturalhoofcare@gmail.com
and consult with your veterinarian.
Part 2. Balancing Equine Diets
Nutritional imbalances can be a factor in many equine health issues, as discussed above and in this article on nutrition basics by Kellon (2014). In this section, we will show you how to balance your horse's diet based on hay and pasture samples from Harrison County, Indiana (Fig. 1).
Nutritional imbalances can be a factor in many equine health issues, as discussed above and in this article on nutrition basics by Kellon (2014). In this section, we will show you how to balance your horse's diet based on hay and pasture samples from Harrison County, Indiana (Fig. 1).
Fig. 1. Google map showing approximate location of hay and pasture samples in Harrison County, Indiana.
The strategy is to determine a base diet from good quality grass hay and/or pasture and then correct for deficiencies and excesses in terms of macronutrients (major elements), micronutrients (trace elements), digestible energy (calories) and crude protein (nitrogen (N) content). The procedure is actually very straightforward and consists of comparing your hay and pasture analysis to the nutrient requirements published by the National Research Council (NRC, 2007) after applying constraints on mineral ratios. These constraints are necessary because of antagonistic effects, i.e., the negative effect of one element (mineral) on the absorption of another mineral. A good example is iron (Fe), which is in excess of NRC requirements in almost all feeds and supplements. Excessive amounts of iron limit the absorption of zinc (Zn), and possibly copper (Cu), and are associated with iron overload, insulin resistance and infection and immunity.
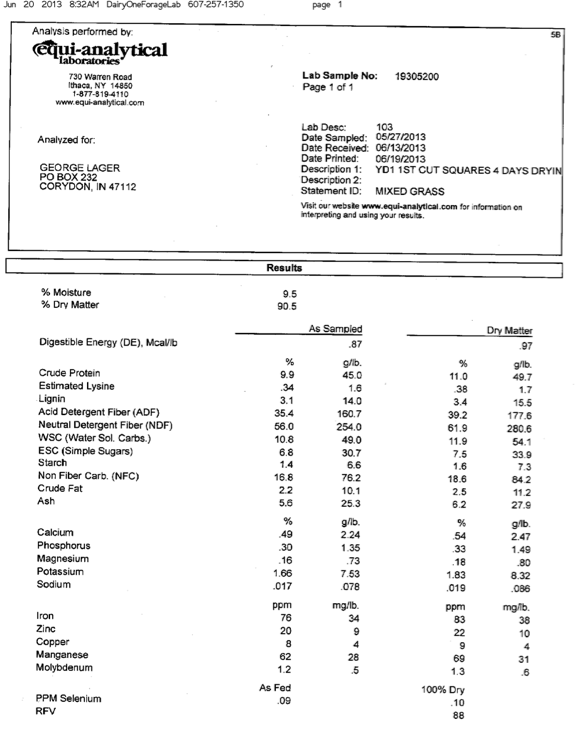
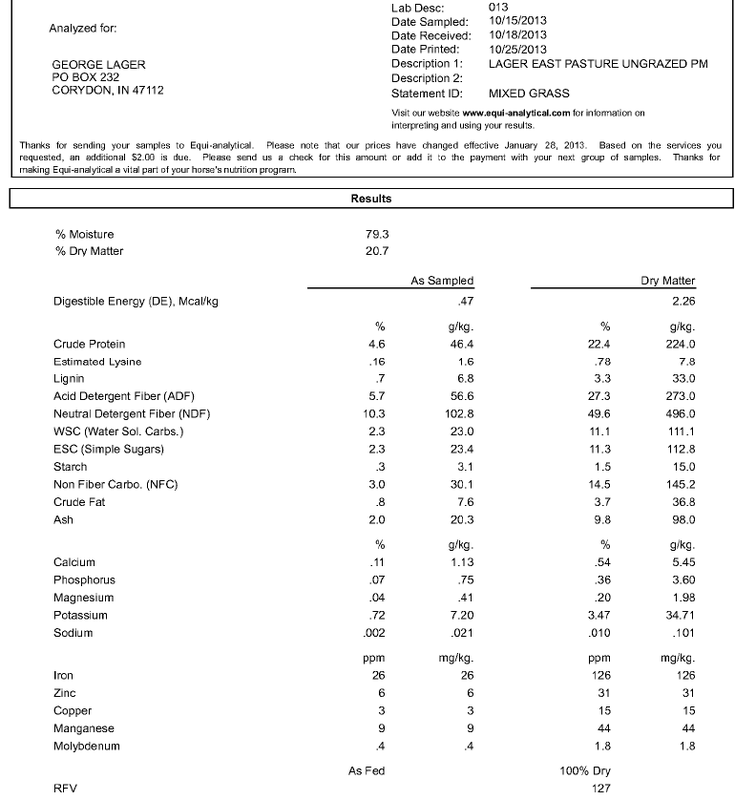
Let’s get started. The first piece of information you will need is the nutrient profile of your grass hay and pasture. All hay and pasture analyses on this page have been determined by Equi-analytical Laboratory, Ithaca, New York, a division of Dairy One. Go to their web site for information on sampling procedures. Table 1 below shows results from Equi-analytical for grass hay and pasture samples collected near Central, Indiana. Hay sample is first cutting baled in May 2013; pasture sample was collected in October 2013.
Complete sample analyses as received from Equi-analytical are attached at the end of this section as Appendix 1. We have also recently uploaded some examples of hay analyses (minerals, crude protein, neutral detergent fiber and digestible energy) from Harrison and adjacent counties in Indiana and Kentucky (Appendix 2).
Let’s get started. The first piece of information you will need is the nutrient profile of your grass hay and pasture. All hay and pasture analyses on this page have been determined by Equi-analytical Laboratory, Ithaca, New York, a division of Dairy One. Go to their web site for information on sampling procedures. Table 1 below shows results from Equi-analytical for grass hay and pasture samples collected near Central, Indiana. Hay sample is first cutting baled in May 2013; pasture sample was collected in October 2013.
Complete sample analyses as received from Equi-analytical are attached at the end of this section as Appendix 1. We have also recently uploaded some examples of hay analyses (minerals, crude protein, neutral detergent fiber and digestible energy) from Harrison and adjacent counties in Indiana and Kentucky (Appendix 2).
For those who prefer not to work through the diet calculations, here's the take-home message.
1. Grass hay and pasture in Harrison County, Indiana are deficient in copper, zinc, sodium and manganese (pasture only). Selenium meets the minimum requirement established by the National Research Council (Nutrient Requirements of Horses, 2007).
2. If these minerals are supplemented at recommended levels, horses at average activity levels (pleasure) and working horses (moderate workload) can be maintained on grass hay and pasture, respectively.
3. High-iron hay and pasture require correspondingly higher levels of supplementation because iron limits the absorption of certain trace minerals, such as zinc.
4. Analyze your hay before purchase and, if possible, choose high-quality hay with relatively low iron content (< 100 ppm).
5. The nutrient content of hay and pasture will vary based on soil type, farming practices and type of grass, or other pasture plants. Therefore, it is important that you balance your horse's diet based on forage from your local area.
Your browser does not support viewing this document. Click here to download the document.
To simplify the calculations, we will consider only two NRC horse classes:
1. Average maintenance, which would apply to most pleasure horses in Harrison County, Indiana (NRC Average Maintenance: average alertness, mood and activity levels on turn out);
2. Moderate work, a workload characteristic of some endurance horses, or horses in training (NRC Moderate Work: 3 to 5 hours per week; 30% walk, 50% trot, 10% canter, 5% low jumping, cutting or other skill work).
Formulae are available from NRC to calculate nutrient requirements but for convenience we will use their online software. Initially, we will also assume that both horses in our calculation weigh 500 kg (1100 lbs) and that both horses are in good health. Table 2 lists their NRC estimated daily nutrient requirements.
1. Average maintenance, which would apply to most pleasure horses in Harrison County, Indiana (NRC Average Maintenance: average alertness, mood and activity levels on turn out);
2. Moderate work, a workload characteristic of some endurance horses, or horses in training (NRC Moderate Work: 3 to 5 hours per week; 30% walk, 50% trot, 10% canter, 5% low jumping, cutting or other skill work).
Formulae are available from NRC to calculate nutrient requirements but for convenience we will use their online software. Initially, we will also assume that both horses in our calculation weigh 500 kg (1100 lbs) and that both horses are in good health. Table 2 lists their NRC estimated daily nutrient requirements.
Your browser does not support viewing this document. Click here to download the document.
You can construct a table showing daily nutrient intake based on your hay and grass analyses using some simple math. Multiply the percentages in Table 1 by 10 to convert to g/kg (grams per kilogram forage). In our example, we are assuming that each horse consumes 2% BW (body weight) per day (0.02 x 500 kg = 10 kg) so the g/kg value determined above is multiplied by 10 to find total dietary intake per day. For example, 0.49% x 10 = 4.9 g Ca/kg forage. For 2% BW, 4.9 g/kg x 10 kg (22 lbs) = 49 g Ca per day. Since concentrations for the trace elements are already expressed in terms of mass units (mg/kg), simply multiply these values by 10. For example, 8 mg/kg Cu x 10 kg = 80 mg Cu per day. The results for our hay and pasture analyses are listed in Table 3.
Your browser does not support viewing this document. Click here to download the document.
Because the water content of grass usually makes up about 70-90% of the total weight of the sample, results for pasture grass are calculated on a dry matter basis (100% dehydrated sample, or 0% moisture). Unfortunately, unlike with hay, we cannot control, or determine, how much grass a horse consumes per day. As a result, the nutrient amounts shown in Table 3 are theoretical values, or educated estimates. A horse on 24/7 turn out would have to consume more grass than the kg dry matter amount in our calculation because of the high moisture content of grass. Refer to the equi-analytical web site for a more detailed discussion of "as sampled" versus "dry matter" results and the pasture analysis in Appendix 1.
At this point, we balance nutrients based on several mineral constraints (Eleanor Kellon, VMD, Equine Nutrition Courses). To compensate for Fe excess, the Fe:Cu ratio is set to 4:1 and the target values for Cu:Zn:Mn to 1:3:3. For the hay analysis, Cu concentration is Fe/4 = 190 and Cu:Zn:Mn = 190:570:570. The corresponding ratio for grass pasture is 315:945:945. Ideally, target values for the ratios Ca/Mg and Ca/P should be within the range 1.5 to 2.0:1. For our hay and grass samples, the unconstrained ratios are Ca/Mg = 3.06, Ca/P = 1.63 and Ca/Mg = 2.7, Ca/P = 1.5, respectively.
Final results are summarized in Table 4 after balancing on Fe and rounding off to the tenths place. In this example, we will assume that the diet of the pleasure horse is grass hay only and the working horse (endurance) is 24/7 pasture.
At this point, we balance nutrients based on several mineral constraints (Eleanor Kellon, VMD, Equine Nutrition Courses). To compensate for Fe excess, the Fe:Cu ratio is set to 4:1 and the target values for Cu:Zn:Mn to 1:3:3. For the hay analysis, Cu concentration is Fe/4 = 190 and Cu:Zn:Mn = 190:570:570. The corresponding ratio for grass pasture is 315:945:945. Ideally, target values for the ratios Ca/Mg and Ca/P should be within the range 1.5 to 2.0:1. For our hay and grass samples, the unconstrained ratios are Ca/Mg = 3.06, Ca/P = 1.63 and Ca/Mg = 2.7, Ca/P = 1.5, respectively.
Final results are summarized in Table 4 after balancing on Fe and rounding off to the tenths place. In this example, we will assume that the diet of the pleasure horse is grass hay only and the working horse (endurance) is 24/7 pasture.
Your browser does not support viewing this document. Click here to download the document.
Interpreting Table 4 results
1. With the exception of Cu and Zn, our grass hay sample provides all of the mineral, protein and calorie requirements for adult horses at average maintenance. The Ca/Mg ratio is slightly high but tolerable. Supplementing with additional Mg to reach our target value (2:1) would require 8.5 g Mg.
2. Working horses (moderate level) grazing our pasture 24/7 require supplementation with Cu, Zn, Mn and possibly Se (not analyzed). The addition of 7 g Mg to the diet would be needed to achieve the 2:1 target value for the Ca/Mg ratio.
3. In addition to the mineral deficiencies noted above, hay and grass pasture in Harrison County, Indiana are particularly low in Na (compare Tables 2 and 3).
4. In cases 1 and 2 above, with the exception of Na and Se, major and trace elements are approximately equal to, or exceed, 150% NRC minimum requirement. This safety margin ensures that we have compensated for metabolic differences among horses as well as inaccuracies in analytical results and sampling errors.
5. A number of grass hays that we have tested have Fe concentrations exceeding 200 ppm. In these cases, balancing on Fe will significantly increase the amount of Cu and Zn required to reach the target ratio 4:1:3:3 for Fe:Cu:Zn:Mn. Analyze hay before purchasing and choose good quality hay with relatively low Fe concentration (< 100 ppm). This will be more cost effective because less Cu/Zn supplementation is required to correct deficiencies.
6. The nutrient profiles of hay and pasture will vary based on soil type, farming practices and type of grass, or other pasture plants. Therefore, it is important that you balance your horse's diet based on forage from your local area.
Keep in mind that balancing software is only a tool and diet must be evaluated on a regular basis in terms of body weight and condition score. For some good photographs with comments by Don Henneke, Ph.D. (author of the Henneke condition scoring system) download this PDF on body condition scores. A short video on the proper way to estimate your horse's weight can be found here.
In Part 3, we will discuss how to choose and formulate your own supplements.
1. With the exception of Cu and Zn, our grass hay sample provides all of the mineral, protein and calorie requirements for adult horses at average maintenance. The Ca/Mg ratio is slightly high but tolerable. Supplementing with additional Mg to reach our target value (2:1) would require 8.5 g Mg.
2. Working horses (moderate level) grazing our pasture 24/7 require supplementation with Cu, Zn, Mn and possibly Se (not analyzed). The addition of 7 g Mg to the diet would be needed to achieve the 2:1 target value for the Ca/Mg ratio.
3. In addition to the mineral deficiencies noted above, hay and grass pasture in Harrison County, Indiana are particularly low in Na (compare Tables 2 and 3).
4. In cases 1 and 2 above, with the exception of Na and Se, major and trace elements are approximately equal to, or exceed, 150% NRC minimum requirement. This safety margin ensures that we have compensated for metabolic differences among horses as well as inaccuracies in analytical results and sampling errors.
5. A number of grass hays that we have tested have Fe concentrations exceeding 200 ppm. In these cases, balancing on Fe will significantly increase the amount of Cu and Zn required to reach the target ratio 4:1:3:3 for Fe:Cu:Zn:Mn. Analyze hay before purchasing and choose good quality hay with relatively low Fe concentration (< 100 ppm). This will be more cost effective because less Cu/Zn supplementation is required to correct deficiencies.
6. The nutrient profiles of hay and pasture will vary based on soil type, farming practices and type of grass, or other pasture plants. Therefore, it is important that you balance your horse's diet based on forage from your local area.
Keep in mind that balancing software is only a tool and diet must be evaluated on a regular basis in terms of body weight and condition score. For some good photographs with comments by Don Henneke, Ph.D. (author of the Henneke condition scoring system) download this PDF on body condition scores. A short video on the proper way to estimate your horse's weight can be found here.
In Part 3, we will discuss how to choose and formulate your own supplements.
Appendix 1. Sample analyses as received from Equi-analytical
Mixed grass hay; square bales, first cutting, drying time 4 days. Mulitply Mcal/lb, g/lb and mg/lb by 2.2 to obtain equivalent values in kg, e.g., Mcal/kg. For hay, diet balancing is based on "As Sampled", rather "Dry Matter" results. This is what you are feeding your horse.
Mixed grass pasture collected in October on mild (High 16 C = 60 F, Low 4 C = 40 F), cloudless day at peak carbohydrate levels (4:00 PM). This same pasture was analyzed in May for primary nutrients (e.g., sugars and starch, crude protein, etc.), which does not include major and trace minerals (see Part 1). Because of the high moisture content of grass, "Dry Matter" results are used in diet calculations with pasture grass.
Appendix 2a and Appendix 2b: Examples of hay analyses from Harrison and adjacent counties comparing mineral concentrations, crude protein, neutral detergent fiber and digestible energy (calories). Several "speciality" hays are included for comparison.
Your browser does not support viewing this document. Click here to download the document.
Your browser does not support viewing this document. Click here to download the document.
Part 3. Choosing and formulating your own mineral supplements
We are now ready to choose mineral supplements and determine the amount required to correct deficiencies in your horse’s diet. Supplements are usually not available as uncombined elements (e.g., Calcium) but occur with other elements in chemical compounds (e.g., calcium carbonate). In our example, we are going to target deficiencies in Cu, Zn and Mn with single ingredients rather than commercial mixtures, which can contain minerals and calories that your horse does not need. In addition, unless commercial products are formulated specifically for your local area, supplement amounts will usually not be correct.
Listed below are supplements recommended by Eleanor Kellon, VMD (Carol Layton, Balanced Equine Nutrition, pers. comm.). It is important to use compounds that are low in iron and easily absorbed in the digestive tract. If possible, avoid antagonistic effects, e.g., iron/zinc and copper/sulfur.
Table 1. Recommended mineral supplements
____________________________________________________
Ca: Calcium chloride, or calcium carbonate.
P: Sodium phosphate or monosodium phosphate. Monocalcium phosphates and dicalcium phosphates are not recommended because of high iron content.
Mg: Magnesium carbonate, if you have excessive amounts of iron in your forage and soil. The oxide MgO can contain 5000 ppm, or 5 mg/g iron. If you prefer to use MgO, purchase high-purity grade. Avoid magnesium sulfate (Epsom salts). It is poorly absorbed and can cause diarrhea.
Cu: Copper polysaccharide (poly copper). Copper sulfate has the potential to cause more interference with copper absorption, especially if your water supply is high in sulfates (i.e., antagonistic effects). It is uncertain whether poly Cu is more bioavailable (absorbable) than the sulfate; however, the poly "coating" (Cu ion weakly bonded within polysaccharide complex) could inhibit Cu precipitation by excess sulfates in the small intestine. Copper sulfate can be used if poly copper is not available.
Zn: Zinc sulfate or zinc polysaccharide (poly zinc). Zn bioavailability is poor with zinc oxide.
Mn: Manganese sulfate.
Na and Cl: Loose white salt, or sea salt, e.g., Redmond salt.
Se: Selenium-enriched yeast (Se replaces Sulfur (S) in amino acids, e.g., selenomethionine). Se-yeast is more bioavailable, better retained and less toxic than inorganic selenium compounds (sodium selenite and sodium selenate).
________________________________________________________
We are now ready to choose mineral supplements and determine the amount required to correct deficiencies in your horse’s diet. Supplements are usually not available as uncombined elements (e.g., Calcium) but occur with other elements in chemical compounds (e.g., calcium carbonate). In our example, we are going to target deficiencies in Cu, Zn and Mn with single ingredients rather than commercial mixtures, which can contain minerals and calories that your horse does not need. In addition, unless commercial products are formulated specifically for your local area, supplement amounts will usually not be correct.
Listed below are supplements recommended by Eleanor Kellon, VMD (Carol Layton, Balanced Equine Nutrition, pers. comm.). It is important to use compounds that are low in iron and easily absorbed in the digestive tract. If possible, avoid antagonistic effects, e.g., iron/zinc and copper/sulfur.
Table 1. Recommended mineral supplements
____________________________________________________
Ca: Calcium chloride, or calcium carbonate.
P: Sodium phosphate or monosodium phosphate. Monocalcium phosphates and dicalcium phosphates are not recommended because of high iron content.
Mg: Magnesium carbonate, if you have excessive amounts of iron in your forage and soil. The oxide MgO can contain 5000 ppm, or 5 mg/g iron. If you prefer to use MgO, purchase high-purity grade. Avoid magnesium sulfate (Epsom salts). It is poorly absorbed and can cause diarrhea.
Cu: Copper polysaccharide (poly copper). Copper sulfate has the potential to cause more interference with copper absorption, especially if your water supply is high in sulfates (i.e., antagonistic effects). It is uncertain whether poly Cu is more bioavailable (absorbable) than the sulfate; however, the poly "coating" (Cu ion weakly bonded within polysaccharide complex) could inhibit Cu precipitation by excess sulfates in the small intestine. Copper sulfate can be used if poly copper is not available.
Zn: Zinc sulfate or zinc polysaccharide (poly zinc). Zn bioavailability is poor with zinc oxide.
Mn: Manganese sulfate.
Na and Cl: Loose white salt, or sea salt, e.g., Redmond salt.
Se: Selenium-enriched yeast (Se replaces Sulfur (S) in amino acids, e.g., selenomethionine). Se-yeast is more bioavailable, better retained and less toxic than inorganic selenium compounds (sodium selenite and sodium selenate).
________________________________________________________
Your browser does not support viewing this document. Click here to download the document.
The relatively high iron (Fe) content of Redmond salt might be of concern since we are trying to minimize the amount of dietary Fe. From the trace element analysis, the minimum and average Fe concentrations are 300 ppm and 500 ppm, respectively. Remember that 1 ppm = 1 mg/kg. For a daily supplement of 2 tablespoons = 1 fl ounce ~ 28 g salt (measured weight), Fe dietary contribution from Redmond salt for the average concentration is only 14 mg (500 mg/kg x 0.028 kg salt). This is a very small amount relative to that for grass hay (760 mg) and pasture (1260 mg) (Table 4, Part 2). In contrast to white salt, Redmond salt is ancient sea salt formed about 200 million years ago at the present location of Utah. Redmond salt (#10 Fine) is available locally from Georgetown Feed, Georgetown, Indiana.
For our Cu/Zn supplements, we chose poly Cu and poly Zn (powder) rather than the sulfate analogs. Table 2 shows the supplement calculation for hay and pasture from Central, Indiana.
Your browser does not support viewing this document. Click here to download the document.
As an example, let’s work through the supplement calculation in scoops using the product label for poly Zn from Uckele Health and Nutrition.
For this product, 0.5 scoop poly Zn = 3 g compound = 670 mg Zn (670 mg/3000 mg x 100 = 22% Zn). Therefore, you would need to add ~0.28 scoop to balance the hay-only diet (0.28/0.5 scoop x 3 g poly Zn = 1.68 g = 1680 mg). For poly Cu, 0.5 scoop = 2.5 g compound = 310 mg Cu (12.4% Cu). In this case, add ~0.18 scoop (0.18/0.5 scoop x 2.5 g poly Cu = 0.9 g = 900 mg). As you can see, it’s impossible to measure out accurate amounts using the “scoop method”. It’s easier and more accurate to use an inexpensive gram scale.
Some labels are more user friendly than others. A good example is this poly Zn label from HorseTech. The "Directions for Use" are very explicit and explain in detail how to calculate the amount of poly Zn compound required to balance the Zn deficiency in Table 2 (click on image and view last paragraph under "Directions for Use"). It's the same procedure given in the footnote to Table 2.
Some labels are more user friendly than others. A good example is this poly Zn label from HorseTech. The "Directions for Use" are very explicit and explain in detail how to calculate the amount of poly Zn compound required to balance the Zn deficiency in Table 2 (click on image and view last paragraph under "Directions for Use"). It's the same procedure given in the footnote to Table 2.
Supplements can be incorporated in wet non-molasses beet pulp as part of the daily ration. Cu and Zn polysaccharides as single ingredients are somewhat bitter. Begin supplementing with small amounts and gradually increase to the recommended level. Initially, you might have to use a flavor enhancer, such as CocoSoya (coconut and soybean oils), until your horse becomes accustomed to the taste of the beet pulp mixture. For forage with very high iron contents (>> 200 ppm), you can reduce the amounts of polysaccharides required and improve palatability by relaxing the 4:1 Fe:Cu. The 4:1 Fe:Cu ratio is ideal but horses can tolerate ratios up to 10:1 (Eleanor Kellon, VMD, Equine Nutrition Courses).
You might be wondering if locally available feeds and mineral supplements compensate for trace mineral deficits in grass hay (Table 2). Table 3 indicates that most popular horse products will not balance Cu and Zn deficiencies in our grass hay example for a 4:1:3 Fe:Cu:Zn ratio.
For hay, or pasture, with higher iron concentrations, mineral deficits for these commercial products will increase. For example, the mineral deficit for Dumor Vitamin Gold will approximately double for our pasture example in Table 2 (126 ppm Fe).
For hay, or pasture, with higher iron concentrations, mineral deficits for these commercial products will increase. For example, the mineral deficit for Dumor Vitamin Gold will approximately double for our pasture example in Table 2 (126 ppm Fe).
Your browser does not support viewing this document. Click here to download the document.
Please consult with your veterinarian, or equine nutritionist, before formulating your own supplements, or changing your horse's diet.
Part 4. Selenium (Se): Comments on NRC requirements and geochemistry
Recommended minimum daily Se intakes in Table 2, Part 2 are 1.0 mg and 1.13 mg per day for horses at average maintenance and moderate workload, respectively. NRC recognizes that these levels might be too low for optimal horse health. In addition, the recommended Se intake for exercising horses at moderate and heavy workloads is only slightly greater than those at maintenance. This small increase does not account for Se losses from red blood cells during exercise and greater excretion (urination) following exercise. As indicated for other minerals, supplement Se at 150% minimum NRC requirement; however, higher Se levels are recommended for working horses, pregnant and lactating mares, and growing horses (Table 1, JD Pagan 2000).
There are always some health concerns about elements like Se that are beneficial in small amounts but have a low threshold of toxicity. The maximum total Se per day recommended by most equine nutritionists is based on Dry Matter Intake (0.3 mg/kg DMI), or body weight (1 mg/500 lbs BW).
Recommended minimum daily Se intakes in Table 2, Part 2 are 1.0 mg and 1.13 mg per day for horses at average maintenance and moderate workload, respectively. NRC recognizes that these levels might be too low for optimal horse health. In addition, the recommended Se intake for exercising horses at moderate and heavy workloads is only slightly greater than those at maintenance. This small increase does not account for Se losses from red blood cells during exercise and greater excretion (urination) following exercise. As indicated for other minerals, supplement Se at 150% minimum NRC requirement; however, higher Se levels are recommended for working horses, pregnant and lactating mares, and growing horses (Table 1, JD Pagan 2000).
There are always some health concerns about elements like Se that are beneficial in small amounts but have a low threshold of toxicity. The maximum total Se per day recommended by most equine nutritionists is based on Dry Matter Intake (0.3 mg/kg DMI), or body weight (1 mg/500 lbs BW).
Let’s assume that you are feeding 16 lbs grass hay containing 0.1 ppm Selenium (Se) plus 2 lbs mineral supplement with Guaranteed Analysis 1.5 ppm Se (product label). To determine total Se in your horse’s diet, multiply ppm concentration (= mg/kg) for each feed by the amount consumed in kg, or lbs (1 kg =2.2 lbs). For the grass hay, 0.1 mg/kg x 16 lbs = 0.1 mg/2.2 lbs x 16 lbs = 0.7 mg Se. Mineral supplement will supply an additional 1.4 mg Se (1.5 mg/2.2 lbs x 2 lbs = 1.4 mg Se) for total Se = 2.1 mg per day. In this case, DMI = 18 lbs, or 8 kg, and maximum recommended Se = 0.3 mg/kg x 8 kg = 2.4 mg.
According to NRC estimates, the maximum tolerable level of Se is 2 mg/kg DMI of daily diet, or in our forage-based diet, 20 mg (2 mg/kg x 10 kg). In our grass hay example, if you add 1 mg Se supplement to meet 200% NRC minimum daily requirement (0.1 mg/kg x 10 kg hay + 1 mg supplement = 2 mg), total Se intake is an order of magnitude (10x) less than the tolerable amount.
The lethal dose of Se is expressed in terms of LD50, which is the amount needed to kill 50% of the population of test animals. The LD50 for Se is 3.3 mg/kg per body weight. For example, a 500 kg (1100 lb) horse would have to consume 1650 mg Se per day (3.3 mg/kg x 500 kg).
To determine the correct supplement amount, you must analyze your hay and pasture, or request whole blood Se tests, which measure Se in both plasma and inside red blood cells.
Geochemical maps showing the Se distribution in soils and stream sediments must be interpreted with caution. Figure 1 shows the National Geochemical Survey Map for Se published by the U.S. Geological Survey (USGS). Harrison County, Indiana and the surrounding area, including the Louisville Metro area, are circled in black. You can also view these results by county.
The lethal dose of Se is expressed in terms of LD50, which is the amount needed to kill 50% of the population of test animals. The LD50 for Se is 3.3 mg/kg per body weight. For example, a 500 kg (1100 lb) horse would have to consume 1650 mg Se per day (3.3 mg/kg x 500 kg).
To determine the correct supplement amount, you must analyze your hay and pasture, or request whole blood Se tests, which measure Se in both plasma and inside red blood cells.
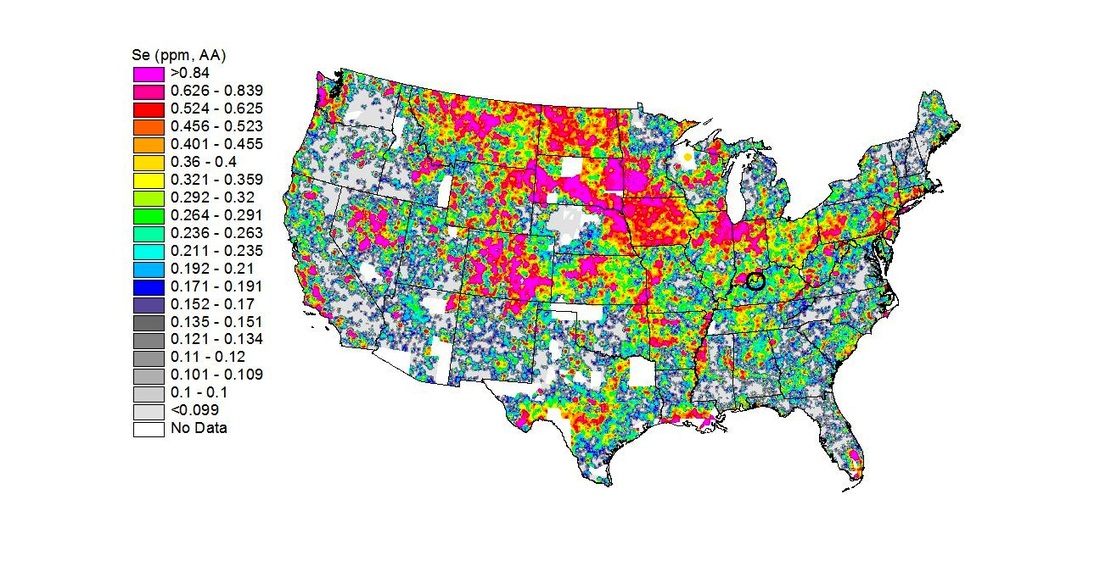
Geochemical maps showing the Se distribution in soils and stream sediments must be interpreted with caution. Figure 1 shows the National Geochemical Survey Map for Se published by the U.S. Geological Survey (USGS). Harrison County, Indiana and the surrounding area, including the Louisville Metro area, are circled in black. You can also view these results by county.
Fig. 1. Selenium (Se) distribution map of the U.S. based on results from Atomic Absorption Spectrometry (AA). Harrison County, Indiana and the surrounding area are circled in black. Average Se concentration for Harrison County based on soil and stream sediment analyses is 0.34 ppm ± 0.05. Click on image for larger version.
After inspecting this map, you might conclude that the Se concentration in your hay/pasture exceeds NRC requirements, i.e., 0.34 ppm soils (or stream sediments derived from soil erosion) versus 0.1 ppm NRC. The dominant Se species in acidic soils of Harrison County, Indiana (pH ~ 5-6) is selenite anion (Se-IV), which is strongly adsorbed (bound) to cation sites on surfaces of inorganic compounds, such as clays and hydrous Fe oxides. As a result, it is very immobile and unavailable for plant uptake (Mayland et al. 1991). In acidic soils, plants more readily absorb (take in) the selenate form (Se-VI). The problem is that analytical results in Fig. 1 measure total Se (selenite plus selenate) rather than the more bioavailable selenate. That’s one reason why the average Se concentration in Harrison County soils (0.34 ppm) is greater than the amount measured in our hay sample (~0.1 ppm). There are too many geochemical and biochemical (related to plant physiology and microbial processes) factors that affect the nutrient profile of plants to use soil analyses for diet balancing.
The mineral content of hays is also affected by farming practices, such as fertilization, time of cutting and number of cut. Most hay producers in this area routinely apply lime (pulverized limestone) to their soils to increase pH to near neutral (pH ~ 7). For soils with relatively low organic content (e.g., Crider series in Harrison County) this practice will increase Se bioavailability (Eich-Greatorex et al. 2007). In a recent Finnish study of low-Se soils, application of sodium selenate in commercial fertilizers to cereal and forage crops benefited both human and animal nutrition, and increased plant yields.
Se supplements are usually combined with vitamin E, another potent antioxidant that works in conjunction with Se. It’s a good combination because vitamin E is always deficient in grass hay and must be supplemented (1000 IU/500 lbs BW) if your horse is not on pasture (E Kane, Advances in Equine Nutrition IV, Kentucky Equine Research, p. 61, 2009).
If you are interested in the geochemistry of selenium, download this short PowerPoint presentation (pdf version) “Selenium in Sediment and Soil” by Jonathan Gillip, USGS. For a general introduction to selenium for horse owners, refer to the article "Selenium: A Balancing Act" at theHorse.com. More information on comparisons between organic and inorganic Se sources and the complementary nature of Se and vitamin E can be found here.
Se supplements are usually combined with vitamin E, another potent antioxidant that works in conjunction with Se. It’s a good combination because vitamin E is always deficient in grass hay and must be supplemented (1000 IU/500 lbs BW) if your horse is not on pasture (E Kane, Advances in Equine Nutrition IV, Kentucky Equine Research, p. 61, 2009).
If you are interested in the geochemistry of selenium, download this short PowerPoint presentation (pdf version) “Selenium in Sediment and Soil” by Jonathan Gillip, USGS. For a general introduction to selenium for horse owners, refer to the article "Selenium: A Balancing Act" at theHorse.com. More information on comparisons between organic and inorganic Se sources and the complementary nature of Se and vitamin E can be found here.
Take-home message
1. Se concentration in most Harrison County, Indiana hays meets minimum NRC requirements (0.1 ppm = 0.1 mg/kg).
2. Se levels higher than 150% NRC are recommended for most horse classes.
3. Total Se from all feed rations is normally in the range of ~2-3 mg per day.
4. Local soil maps are poor indicators of Se bioavailability and cannot be used for diet balancing.
5. Supplement amounts must be determined from hay/pasture analyses, or whole blood tests.
1. Se concentration in most Harrison County, Indiana hays meets minimum NRC requirements (0.1 ppm = 0.1 mg/kg).
2. Se levels higher than 150% NRC are recommended for most horse classes.
3. Total Se from all feed rations is normally in the range of ~2-3 mg per day.
4. Local soil maps are poor indicators of Se bioavailability and cannot be used for diet balancing.
5. Supplement amounts must be determined from hay/pasture analyses, or whole blood tests.
Part 5. Biochemical function of deficient minerals in Harrison County, Indiana hay/pasture
We now have a balanced mineral diet and know what minerals must be supplemented. A fair question to ask at this point is “How important are these supplements to horse health?” There is an extensive amount of literature on the role of minerals in equine diets. You can refer to several of the links provided above for more details, or link to Dr. Kellon's blog. Rather than present a comprehensive literature review, we have attempted to summarize this information in Table 1. Table 1 is not all-inclusive but illustrates some of the primary functions of minerals deficient in Harrison County, Indiana hay and pasture, and symptoms associated with deficiencies. Manganese (Mn) has been omitted because of the limited nature of our database on pasture grass. None of the hays tested were Mn-deficient.
Your browser does not support viewing this document. Click here to download the document.
Part 6. Iodine deficiency and supplement analysis
Table 1 includes one additional mineral in our list of deficient minerals in Harrison County, Indiana hay and pasture. The mineral iodine, which is critical to thyroid function, is not included in the nutrient profile provided by forage labs because the analysis is difficult and expensive. However, most soils are deficient in iodine, which is the reason why iodized salt is recommended in human diets. Iodine levels can be determined indirectly with a full thyroid panel, which includes blood T4 (levothyroxine) and T3 (triiodothyronine) hormone levels.
Table 1 includes one additional mineral in our list of deficient minerals in Harrison County, Indiana hay and pasture. The mineral iodine, which is critical to thyroid function, is not included in the nutrient profile provided by forage labs because the analysis is difficult and expensive. However, most soils are deficient in iodine, which is the reason why iodized salt is recommended in human diets. Iodine levels can be determined indirectly with a full thyroid panel, which includes blood T4 (levothyroxine) and T3 (triiodothyronine) hormone levels.
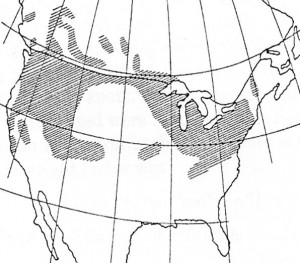
The major contribution to iodine in soils is from the volatilization of iodine from the ocean surface, e.g., vapor emissions from algae (Iodine and Inorganic Iodides: Human Health Aspects, Risher, JF and Keith, S, ed., World Health Organization, 2009). In general, there is a good correlation between iodine concentration versus distance from the ocean. Because soils form by weathering (physical and chemical decomposition) of underlying rocks, the chemical composition of parent rock can also affect the concentration of iodine in soils. For example, soils formed from igneous and metamorphic rocks (e.g., granite, volcanic rock and gneiss) are generally lower in iodine than those formed from sedimentary rocks (e.g., limestones and sandstones). This accounts for the lower iodine concentrations in the Upper Midwest and Great Lakes region (“Goiter Belt”), and the Northeast and Northwest U.S. (Fig. 1).
Fig. 1. Iodine deficient soils (hachured) in the U.S. The Upper Midwest and Great Lakes region form what is known as "The Goiter Belt". Source: Salt Institute
Possible iodine supplements include iodized (with potassium iodide spray) table salt (e.g., grocery store salt), calcium iodate, organic iodine (as opposed to inorganic forms, such as potassium iodide) in the form of ethylenediamine hydroiodide (EDDI), ocean kelp and naturally occurring ancient sea salts, such as Redmond salt.
Let’s take a closer look at each of these supplements.
Iodized Salt
1. The average iodine content for all U.S. brands of iodized salt is 47.5 mg/kg +/- 18.5 mg/kg, i.e., iodine content ranges between 66 and 29 mg/kg, or 66 and 29 ppm. The ppm values correspond to 1.84 mg/ounce salt (66 mg/kg x 0.028 kg = 1.84 mg/ounce) and 0.81 mg/ounce salt (29 mg/kg x 0.028 kg = 0.81 mg/ounce) (Dasgupta et al. 2008).
2. About 2.5-3 ounces of iodized salt at the average concentration (47.5 mg/kg x 0.028 kg = 1.3 mg/ounce) would be needed to satisfy the minimum NRC requirement for iodine in our grass hay example (3.5 mg per day, Nutrient Requirements of Horses, 2007). The minimum NRC requirement for sodium at average maintenance is 10 grams (Table 2, Part 2), or 26 grams (about 1 ounce) supplemented as sodium chloride (remember that sodium chloride consists of 39% sodium and 61% chloride). In other words, using iodized salt for our sodium supplement will not satisfy the iodine requirement.
3. Mineral supplements formulated for this part of the U.S. contain about 1.5 mg iodine per ounce, e.g., VMAX, McCauley's Bros., Versailles, Kentucky). Horse feeds, such as Tribute Kalm ‘N EZ®, provide about 2-4 mg iodine per day for the average idle horse, depending on amount fed.
4. Iodized salt is highly refined and contains small amounts of dextrose (sugar) as a stabilizer to prevent iodine loss. If iodized salt is exposed to moist air during storage (no light), iodide in the form of potassium iodide is converted (oxidized) to iodine, which is subsequently lost to sublimation (direct transition from solid to gaseous state, e.g., snow to water vapor). Based on the study by Dasgupta et al. (2008), iodized salt will loose about 50%-60% of iodine content after 10 days for relative humidity (RH) levels between about 80% and 43 +/- 5% (ambient laboratory RH).
5. Because of its chemical instability, potassium iodide is not commonly used in animal feed. A good inorganic alternative is calcium iodate, which is very stable and contains about the same iodine percent as potassium iodide (64% versus 68% iodine).
Organic iodine (EDDI) (VEDCO)
1. Common iodine supplement for livestock. Contains a minimum of 3.65% iodine, or 37 g/kg = 37,000 mg/kg = 37,000 ppm. No maximum concentration given. Sold in 1 lb bags.
2. About 3 ounces EDDI mixed in 50 lb bag of loose white salt, or Redmond salt, provides ~4 mg iodine per ounce based on minimum concentration.
3. Requires mixing at feed mill (400 lbs minimum at local mills) to achieve homogeneous composition unless you have your own small mixer.
Ocean-K (dried kelp) (Uckele Health and Nutrition)
1. Minimum and maximum iodine levels are 0.05% and 0.08% (guaranteed analysis). Recommended feeding directions 0.5 to 2 scoops (1 scoop = 10 grams kelp).
2. Iodine in the form of dried kelp with other ingredients, such as sea salt, calcium, phosphorous, and potassium.
3. At maximum iodine concentration (0.08%), 1 scoop kelp (10 grams) provides
0.08% = 0.8 g /kg = 0.8 g/kg x 0.010 kg = 0.008 g = 8 mg.
At minimum concentration (0.05%),
0.05% = 0.5 g/kg = 0.5 g/kg x 0.010 kg = 0.005 g = 5 mg.
4. If you are concerned about supplementing iodine at levels slightly above the minimum NRC requirement (3.5 mg per day), supplement with smaller amounts assuming maximum concentration.
For example,
At maximum iodine concentration for 0.5 scoop (5 grams),
0.08% = 0.8 g/kg = 0.8 g/kg x 0.005 kg = 0.004 g = 4 mg.
This scoop amount will also provide 4.5% salt (sodium chloride, maximum), equivalent to 45 g/kg x 0.005 kg = 0.225 g NaCl (21 g NaCl required to meet minimum NRC requirement in our hay forage example in Table 2, Part 3).
Dried Kelp (HorseTech)
1. Minimum iodine concentration is 300 ppm (300 mg/kg = 0.3 mg/gram). Guaranteed analysis. No maximum concentration given.
2. Iodine in the form of dried kelp with potassium and sodium chloride.
3. 1 scoop holds ~5 grams dried kelp containing 1.5 mg iodine (0.3 mg/g x 5 g = 1.5 mg) at the minimum concentration. About 2.5 scoops required to satisfy minimum NRC requirement.
Redmond salt
1. Minimum and average iodine concentrations are 10 and 12 ppm, respectively. No maximum concentration given (refer to trace element analysis of Redmond salt in Part 3).
2. Supplementing with 1 ounce (28 grams) per day at the average concentration, 12 ppm iodine = 12 mg/kg x 0.028 kg = 0.34 mg, an order of magnitude (10x) less than the minimum NRC requirement for iodine (3.5 mg per day).
Take-home message
1. Minerals deficient in Harrison County, Indiana hay and pasture are extremely important to horse health.
2. Iodine is deficient in most soils and must be supplemented.
3. Iodine concentrations in hay are not determined by forage labs because the analytical methods required are difficult and expensive.
4. Iodine levels can be determined indirectly with a blood test (full thyroid panel).
4. Three possible iodine supplements are iodized salt, ethylenediamine hydroiodide (EDDI), and ocean kelp. Each of these supplements has advantages and disadvantages in terms of chemical stability, amount required, specific information on maximum iodine content and preparation.
5. Redmond salt does not provide the NRC minimum iodine requirement for adult horses at maintenance level.
1. Minerals deficient in Harrison County, Indiana hay and pasture are extremely important to horse health.
2. Iodine is deficient in most soils and must be supplemented.
3. Iodine concentrations in hay are not determined by forage labs because the analytical methods required are difficult and expensive.
4. Iodine levels can be determined indirectly with a blood test (full thyroid panel).
4. Three possible iodine supplements are iodized salt, ethylenediamine hydroiodide (EDDI), and ocean kelp. Each of these supplements has advantages and disadvantages in terms of chemical stability, amount required, specific information on maximum iodine content and preparation.
5. Redmond salt does not provide the NRC minimum iodine requirement for adult horses at maintenance level.
Part 7. Interpreting well water analyses in Indiana karst (limestone) terranes
The quality of your livestock water source can adversely affect horse health, particularly in rural areas, or barns, with private groundwater wells, which are not regulated by the Safe Water Drinking Act. In this case, it is your responsibility to test the water and monitor groundwater quality.
Examples of groundwater contaminants include potentially toxic minerals (lead, arsenic, cadmium), volatile organics (solvents, fuel components, such as benzene and toluene), inorganic salts (sulfates, nitrates), agricultural chemicals (pesticide and herbicide residues from runoff) and pathogenic organisms from septic systems and manure piles. Refer to the contaminant table published by U.S. Geological Survey for more information on sources and potential health effects of specific contaminants.
In terms of horse nutrition, high concentrations of dissolved minerals in groundwater, such as iron, calcium, magnesium and sulfate (sulfur as SO4) can be important in balancing your horse’s diet.
In this section, we discuss results from a recent analysis of our farm water, which is drawn from a limestone aquifer (St. Louis Limestone, Blue River Group) in the Mitchell Plain (Plateau) near Corydon, Indiana. The Mitchell Plain is one of two important karst terranes in southern Indiana. Read more about karst at the Indiana Geological Survey web site.
Water samples were collected in August 2014 as part of the Indiana State Ground Water Monitoring Network (Indiana Department of Environmental Management, IDEM). Concentrations of over 400 chemical substances were measured at no cost to homeowners and included in a statewide database on groundwater aquifers, which are very susceptible to surface pollution in karst terranes (Unterreiner, 2006, Bulletin 40, Indiana Department of Natural Resources, Water Division).
Sample results are listed below in Table 1. Only those chemical substances above detectable levels are tabulated. No organic compounds, or toxic minerals, were detected. The table is divided into two sections: General Chemistry, which includes Alkalinity, Chloride and Sulfate, and Metals and Minerals. Results are reported in units of milligrams per liter (mg/L), or micrograms per liter (ug/L). For water, 1 mg/L and 1 ug/L are approximately equivalent to one part per million (ppm) and 1 part per billion (ppb), respectively.
Since this well also provides our domestic water supply, we normally test our water on an annual basis. Results as 06.2014 are: bacterial contamination (negative), nitrates (<0.01 mg/L) and sulfates (13 mg/L).
The property Alkalinity listed under General Chemistry is a measure of the capacity of water to neutralize acids. Groundwater in contact with limestone (a carbonate rock) is concentrated in alkaline compounds such as bicarbonate (baking soda is a type of bicarbonate) and carbonate (CaCO3). These compounds remove hydrogen ions (H+) from the water, neutralizing acids and preventing large changes in pH (measure of H ion concentration; pH = 7 neutral, pH < 7 acidic, pH > 7 alkaline). This is the reason why the pH of ponds and lakes in this area is near neutral (pH = 7) in spite of acidic pollution from rainfall.
Alkalinity in limestone areas is closely related to water hardness because calcium and magnesium, the two minerals that make water “hard”, form carbonate compounds (CaCO3 and MgCO3) in limestone. When limestone comes in contact with groundwater, these compounds are dissolved concentrating calcium and magnesium in the water. Water hardness is generally expressed in terms of CaCO3 concentration and is considered “very hard” in this area (297 mg/L, or ppm, CaCO3).
Contaminant levels in Table 1 (human standards) are given as either Maximum Contaminant Levels (MCLs), or Secondary Maximum Contaminant Levels (SMCLs). The former is associated with adverse health effects whereas the latter is based on aesthetics, such as color and odor, and does not pose a health risk. The Environmental Protection Agency (EPA) establishes both levels.
Livestock drinking standards are compared to human standards and summarized in this table (Summary Recommendations Oetzel) published by the School of Veterinary Medicine, University of Wisconsin.
Examples of groundwater contaminants include potentially toxic minerals (lead, arsenic, cadmium), volatile organics (solvents, fuel components, such as benzene and toluene), inorganic salts (sulfates, nitrates), agricultural chemicals (pesticide and herbicide residues from runoff) and pathogenic organisms from septic systems and manure piles. Refer to the contaminant table published by U.S. Geological Survey for more information on sources and potential health effects of specific contaminants.
In terms of horse nutrition, high concentrations of dissolved minerals in groundwater, such as iron, calcium, magnesium and sulfate (sulfur as SO4) can be important in balancing your horse’s diet.
In this section, we discuss results from a recent analysis of our farm water, which is drawn from a limestone aquifer (St. Louis Limestone, Blue River Group) in the Mitchell Plain (Plateau) near Corydon, Indiana. The Mitchell Plain is one of two important karst terranes in southern Indiana. Read more about karst at the Indiana Geological Survey web site.
Water samples were collected in August 2014 as part of the Indiana State Ground Water Monitoring Network (Indiana Department of Environmental Management, IDEM). Concentrations of over 400 chemical substances were measured at no cost to homeowners and included in a statewide database on groundwater aquifers, which are very susceptible to surface pollution in karst terranes (Unterreiner, 2006, Bulletin 40, Indiana Department of Natural Resources, Water Division).
Sample results are listed below in Table 1. Only those chemical substances above detectable levels are tabulated. No organic compounds, or toxic minerals, were detected. The table is divided into two sections: General Chemistry, which includes Alkalinity, Chloride and Sulfate, and Metals and Minerals. Results are reported in units of milligrams per liter (mg/L), or micrograms per liter (ug/L). For water, 1 mg/L and 1 ug/L are approximately equivalent to one part per million (ppm) and 1 part per billion (ppb), respectively.
Since this well also provides our domestic water supply, we normally test our water on an annual basis. Results as 06.2014 are: bacterial contamination (negative), nitrates (<0.01 mg/L) and sulfates (13 mg/L).
The property Alkalinity listed under General Chemistry is a measure of the capacity of water to neutralize acids. Groundwater in contact with limestone (a carbonate rock) is concentrated in alkaline compounds such as bicarbonate (baking soda is a type of bicarbonate) and carbonate (CaCO3). These compounds remove hydrogen ions (H+) from the water, neutralizing acids and preventing large changes in pH (measure of H ion concentration; pH = 7 neutral, pH < 7 acidic, pH > 7 alkaline). This is the reason why the pH of ponds and lakes in this area is near neutral (pH = 7) in spite of acidic pollution from rainfall.
Alkalinity in limestone areas is closely related to water hardness because calcium and magnesium, the two minerals that make water “hard”, form carbonate compounds (CaCO3 and MgCO3) in limestone. When limestone comes in contact with groundwater, these compounds are dissolved concentrating calcium and magnesium in the water. Water hardness is generally expressed in terms of CaCO3 concentration and is considered “very hard” in this area (297 mg/L, or ppm, CaCO3).
Contaminant levels in Table 1 (human standards) are given as either Maximum Contaminant Levels (MCLs), or Secondary Maximum Contaminant Levels (SMCLs). The former is associated with adverse health effects whereas the latter is based on aesthetics, such as color and odor, and does not pose a health risk. The Environmental Protection Agency (EPA) establishes both levels.
Livestock drinking standards are compared to human standards and summarized in this table (Summary Recommendations Oetzel) published by the School of Veterinary Medicine, University of Wisconsin.
Your browser does not support viewing this document. Click here to download the document.
The iron and sulfate concentrations in our well water are very low, which is good news because these substances have a negative (antagonistic) effect on the absorption and bioavailability of other minerals in your horse feed. We mentioned earlier that iron limits the absorption of both copper and zinc and sulfate can interfere with the absorption of copper.
The relatively high concentrations of calcium and magnesium will add a small but significant amount of these minerals to your horse’s diet. To find out how much, let’s assume that your horse drinks on average about 7 gallons of water per day (26 liters). For calcium, 58 mg/L x 26 L = 1508 mg = 1.5 g. For magnesium, 37 mg/L x 26 L = 962 mg ~ 1 g. Adding these amounts to your diet calculation will account for the mineral contribution from drinking water.
The take-home message from all of this is that you need to test your water source as well as your forage. In karst terranes, it is important to sample at different times of the year to evaluate seasonal changes in groundwater quality.
For Indiana horse owners, we recommend that you take advantage of the groundwater monitoring program offered by IDEM. It’s free and you can obtain valuable information about water chemistry and how it might affect horse health and nutrition.
The relatively high concentrations of calcium and magnesium will add a small but significant amount of these minerals to your horse’s diet. To find out how much, let’s assume that your horse drinks on average about 7 gallons of water per day (26 liters). For calcium, 58 mg/L x 26 L = 1508 mg = 1.5 g. For magnesium, 37 mg/L x 26 L = 962 mg ~ 1 g. Adding these amounts to your diet calculation will account for the mineral contribution from drinking water.
The take-home message from all of this is that you need to test your water source as well as your forage. In karst terranes, it is important to sample at different times of the year to evaluate seasonal changes in groundwater quality.
For Indiana horse owners, we recommend that you take advantage of the groundwater monitoring program offered by IDEM. It’s free and you can obtain valuable information about water chemistry and how it might affect horse health and nutrition.
Copyright George Lager, 2011-2020. All Rights Reserved (®)