In Making Sense of Feed Labels, Part 1: Balancing trace mineral deficiencies in your goat’s diet we learned how to determine if your mineral supplement balances deficiencies in trace minerals, such as copper, zinc and selenium. Daily mineral requirements for goats were based on diets for large breed diary cattle adjusted for differences in body weight. We will use the same approach in balancing the major minerals listed in Table 1. Guaranteed Analysis Mineral/Vitamin Supplement. Read the full length article at Hoegger Farmyard.
Making Sense of Feed Labels: Part 1. Balancing Trace Minerals in Your Goat’s Diet
You are at the feed store looking for a mineral supplement for your goats because you have heard that grass and hay in your area are deficient in minerals. You read the label of a recommended supplement with a “guaranteed analysis” expressed in terms of % (percent), ppm (parts per million), IU (International Units) and USP (U.S. Pharmacopeia) and have absolutely NO idea how all these numbers relate to your goat’s diet (Table 1. Guaranteed Analysis Mineral/Vitamin Supplement). Sound familiar!
To read the full-length article, link to the blog site at Hoegger Farmyard.
Pasture Weeds as Mineral Sources for Goats
The following article was originally published in the November 2013 newsletter from Hoegger Goat Supply (Hoegger Farmyard).
Introduction
There have been several blog articles published recently at Hoegger Farmyard that deal with the problem of copper (Cu) deficiencies in goats. As mentioned, this deficiency can be either primary, or secondary. The former is due to low Cu content of forage whereas the latter reflects the interaction between Cu and other chemical elements (minerals), such as iron (Fe), molybdenum (Mo) and sulfur (S). For these reasons, goats are usually fed Cu supplements to avoid a number of symptoms ranging from loss of pigmentation in hair coat to life-threatening anemia.
Copper deficiency is more pronounced in some regions than others and depends, in part, on the chemical composition of soil and underlying rocks. For example, on our farm in the karst limestone region of southern Indiana (Mitchell Plain), Cu concentrations in grass hay (~8 ppm) and commercial feed (~25 ppm) are adequate to meet the nutritional requirements of our dairy goats (ppm = parts per million, refer to Table 2). In contrast, high Cu supplementation (~1500 ppm Cu bolus) is required in the Adirondack Mountains (upper New York State) where thin, nutrient-poor soils have formed from glacial debris (tills) (refer to blog article by Rose Bartiss on copper deficiency in goats at Hoegger Farmyard).
In nutrient-poor soils, the roots of pasture plants, such as grasses and weeds, have some ability to selectively absorb and concentrate essential minerals. One interesting example from crop science is ragweed, which can have Zn concentrations seven times greater than those of corn leaves at tassel! In other words, as most gardeners know, weeds can deplete soils of nutrients. Goats are great “weedeaters”, so why not use organized plots of certain pasture weeds to supplement minerals in their diet? The trick is to find the right weeds. You want the nutritive value but you don’t want to propagate a host of noxious weeds that will upset your neighbors and the local extension agent.
Our pastures consist of a variety of weeds, including chicory, dandelion, broadleaf dock, common lambsquarters, common and giant ragweed, narrow-leaved (buckhorn) plantain, bull thistle, redroot pigweed, Sericea lespedeza and Jerusalem artichoke (http://oak.ppws.vt.edu/weedindex.htm). We have seen our goats browse all of these weeds until heavy frost near the end of October. We decided to determine macronutrients and micronutrients for some of the more palatable (and controllable) weeds and see if we could find several good candidates for our pasture plots. Let’s face it, goats are browsers and were not meant to graze grass pastures. Their feeding habits and nutritional requirements are more similar to deer than to other domestic ruminants.
Background
The idea to use weeds as alternative pasture plants is not new. Harrington et al. (2006) (http://www.nzpps.org/journal/59/nzpp_592610.pdf) briefly reviewed the pertinent literature and determined the mineral composition of several of the weeds noted above. The weeds were sampled from both conventional and organic plots on the Tokomaru silt loam (soil texture with high proportion of silt to sand and clay) within the Dairy Cattle Research Unit of Massey University, Palmerston North, New Zealand. Both plots were fertilized within the last 12 months (refer to Harrington et al. for more details). Results indicated that common weeds have elevated concentrations of several different nutrients relative to conventional pasture forage, such as perennial ryegrass and white clover.
Methodology
The weeds in our study were collected in the same pasture areas as those browsed by goats. Pasture soils are predominantly silt loams (Crider series), which developed from weathered limestone (calcium carbonate) and loess (windblown sediment). Only the upper ~15 cm (6 in) of each plant was included in the samples (~100 g = 0.22 lb), mimicking as closely as possible the feeding habits of our goats. At this time of year (August-September), some of the weeds had very fibrous stalks with seed heads, e.g., ragweed, lambsquarters and pigweed. With the exception of chicory, only one sample of each weed was collected (no replicates). Chicory was sampled twice to evaluate the nutrient distribution between basal leaf rosette (vegetative state) and flowering stalk (reproduction stage). Hay samples consisted of cores taken from 12-20 representative bales. No fertilizers other than composted horse manure were applied to pastures (www.mitchellplainfarm.com/on-farm-manure-and-mortality-composting.html). Samples were analyzed by the Dairy One Forage Laboratory, Ithaca, New York, using inductively coupled plasma mass spectrometry (ICP-MS).
Results and Discussion
Some of the results from Harrington et al. (2006) and our new analyses of weed and hay samples are compared below in Tables 1 and 2. Note that the unusually high Na concentrations in the New Zealand data are due to atmospheric deposition of sea salt. Several of the weeds analyzed in this study are enriched in both macronutrients and micronutrients relative to grass and legume hays, consistent with the study by Harrington et al. (2006). For example, Ca in ragweed and Jerusalem artichoke is ~50% higher than that in alfalfa–rich hay (Table 1). Zinc, the most deficient micronutrient next to Cu, is enriched in all weeds with the exception of Sericea lespedeza and redroot pigweed (Table 2). The highest Cu concentrations are found in ragweed, chicory and Jerusalem artichoke. These weeds are also good sources of P, Mg and K.
Pigweed, which is a very popular browse among our goats, has about two times the P and Mg content as alfalfa-rich hay. However, pigweed has the potential to accumulate high nitrate (NO3–) concentrations during extreme environmental conditions, such as drought. After ingestion, nitrate is converted to nitrite (NO2-), which interferes with hemoglobin’s ability to carry oxygen to tissues. The nitrate concentration measured for pigweed in this study was ~0.25% (2500 ppm), which is below the maximum level recommended for cattle (<0.6%) (Radostits et al., Veterinary Medicine, 9th ed., 2000). Relatively high nitrate concentrations can also occur in lambsquarters, a nutritious source of Mg, K and Mn. Because goats are browsers and there is a large diversity of weeds in our pastures, they don’t spend much time feeding on one plant. As a result, they can avoid potential toxic effects and still make use of nutrients in both pigweed and lambsquarters.
Sericea lespedeza is high-tannin forage that has been shown to be effective in controlling internal parasites in goats (http://www.scsrpc.org/SCSRPC/Files/sericea_lespedeza.pdf). It’s not a favorite of our goats but they will browse the tops when confined to pastures dominated by this weed. Lespedeza is a legume and, with the exception of Fe, has a nutrient profile very similar to the alfalfa-rich hay in Tables 1 and 2.
Keep in mind that the concentrations of plant nutrients do not necessarily reflect the actual amount absorbed by goats because of antagonistic effects, i.e., the negative effect of one mineral on another. For example, Mo and S can form insoluble compounds with dietary Cu in the rumen that “lock up” Cu and limit its absorption (www.dairygoatjournal.com/87-3/coppers_role_in_goat_health/).
Finally, let’s determine what weeds are best suited for our pasture plots. Ragweed is chock-full of nutrients but it is not a plant you want to propagate in your pasture. The alternative is to temporarily fence goats in ragweed areas, a common practice by goat owners. A similar strategy can be used for pigweed and lambsquarters. Sericea lespedeza is prolific in this area and is the major goat forage in one of our pastures.
The best choices for cultivation based on our study and the results of Harrington et al. (2006) are dandelion, chicory, narrow-leaved plantain and Jerusalem artichoke, all of which are good sources of Cu and Zn. Cu and Zn concentrations in chicory are highest when the plant is grazed in the vegetative state during leaf growth stage (Table 2). Although the leaves were sampled for analysis, tubers of Jerusalem artichoke are particularly tasty to animals (and humans) and enriched in vitamins and minerals. If you are going to expend all of this effort, why not plant weeds that are good for both you and your goats!
It is important to remember that this is a preliminary study. A more systematic, scientific approach with replicate samples would be needed to fully evaluate the use of weeds as nutritional supplements for goats. The nutrient concentrations in Tables 1 and 2 are specific to our local area and will vary depending on soil type, pasture fertilization and maturity stage of the weeds sampled.
Table 1. Macronutrients (% dry matter) in hays and weeds from Harrington et al. (2006) and samples collected near Central, Indiana. Values highlighted in bold are significantly higher than perennial ryegrass or white clover (statistical analyses by Harrington et al.).
|
Hay and Weed Samples from Harrington et al. (2006) |
||||||
|
% |
Ca |
P |
Mg |
K |
Na |
S |
|
Perennial ryegrass |
0.42 |
0.37 |
0.173 |
3.80 |
0.182 |
0.347 |
|
White Clover |
1.19 |
0.347 |
0.237 |
2.83 |
0.205 |
0.213 |
|
Chicory |
1.18 |
0.663 |
0.393 |
3.80 |
0.591 |
0.627 |
|
Narrow-leaved plantain |
1.77 |
0.480 |
0.253 |
1.97 |
0.618 |
0.530 |
|
Broad-leaved dock |
0.80 |
0.430 |
0.520 |
4.10 |
0.026 |
0.287 |
|
Californian thistle |
1.87 |
0.357 |
0.307 |
2.93 |
0.047 |
0.570 |
|
Dandelion |
0.96 |
0.570 |
0.353 |
3.43 |
0.420 |
0.393 |
Hay and Weed Samples Collected near Central, Indiana
|
% |
Ca |
P |
Mg |
K |
Na |
S |
|
Gamagrass |
0.41 |
0.26 |
0.20 |
1.37 |
0.016 |
0.18 |
|
Alfalfa (70%)/orchard grass |
1.38 |
0.21 |
0.31 |
1.58 |
0.009 |
n/a |
|
Ragweed (Giant) |
2.14 |
0.40 |
0.40 |
3.59 |
0.004 |
0.52 |
|
Lambsquarters |
1.28 |
0.30 |
0.50 |
6.60 |
<0.001 |
0.37 |
|
Chicory (flower stalks) |
1.28 |
0.38 |
0.26 |
2.08 |
0.020 |
0.31 |
|
Chicory (basal leaf rosette) |
1.49 |
0.55 |
0.32 |
4.65 |
<0.001 |
0.66 |
|
Narrow-leaved plantain |
1.86 |
0.29 |
0.25 |
3.26 |
0.003 |
0.45 |
|
Sericea lespedeza |
1.31 |
0.21 |
0.18 |
1.31 |
<0.001 |
0.19 |
|
Jerusalem artichoke (leaves) |
2.20 |
0.38 |
0.43 |
3.37 |
<0.001 |
0.32 |
|
Redroot pigweed |
1.28 |
0.49 |
0.63 |
3.22 |
0.002 |
0.24 |
___________________________________________
Note: To convert % to mass multiply by 10, e.g., gamagrass contains 0.41 % Ca = 4.1 g/kg Ca = 4.1 g/2.2 lbs Ca (3 kg hay = 6.6 lbs = 12.3 g Ca). Interpretation of chemical symbols: Ca (calcium), P (Phosphorous), Mg (Magnesium), K (Potassium), Na (Sodium), S (sulfur); n/a (not analyzed).
Table 2. Micronutrients (% dry matter) in hays and weeds from Harrington et al. (2006) and samples collected near Central, Indiana. Values highlighted in bold are significantly higher than perennial ryegrass or white clover (statistical analyses by Harrington et al.).
|
Hay and Weed Samples from Harrington et al. (2006) |
|||||
|
ppm = mg/kg |
Fe |
Cu |
Zn |
Mn |
Mo |
|
Perennial ryegrass |
151 |
7.9 |
22.0 |
99 |
0.64 |
|
White clover |
109 |
8.6 |
22.0 |
55 |
0.22 |
|
Chicory |
167 |
18.6 |
57.7 |
161 |
0.42 |
|
Narrow-leaved plantain |
182 |
15.1 |
37.7 |
109 |
0.27 |
|
Broad-leaved dock |
95 |
7.6 |
30.7 |
283 |
0.42 |
|
Californian thistle |
139 |
17.0 |
41.7 |
120 |
0.21 |
|
Dandelion |
115 |
14.2 |
37.0 |
93 |
0.373 |
Hay and Weed Samples Collected near Central, Indiana
|
ppm = mg/kg |
Fe |
Cu |
Zn |
Mn |
Mo |
|
Gamagrass |
239 |
8 |
25 |
57 |
1.6 |
|
Alfalfa (70%)/orchard grass |
218 |
10 |
20 |
70 |
0.7 |
|
Ragweed (Giant) |
144 |
16 |
72 |
79 |
0.5 |
|
Lambsquarters |
75 |
5 |
32 |
204 |
0.4 |
|
Chicory (flower stalks) |
90 |
12 |
41 |
24 |
0.8 |
|
Chicory (basal leaf rosette) |
117 |
18 |
72 |
36 |
0.8 |
|
Narrow-leaved plantain |
106 |
13 |
46 |
38 |
0.6 |
|
Sericea lespedeza |
98 |
10 |
27 |
65 |
0.8 |
|
Jerusalem artichoke (leaves) |
252 |
27 |
104 |
79 |
0.6 |
|
Redroot pigweed |
132 |
6 |
28 |
46 |
2.1 |
_____________________________________________
Note: ppm = parts per million where 1 ppm = 1 mg/kg, or 1 mg/2.2 lbs, e.g., 3 kg (6.6 lbs) gamagrass contains 8 mg Cu/2.2 x 6.6 = 24 ppm Cu. Interpretation of chemical symbols: Fe (Iron), Cu (Copper), Zn (Zinc), Mn (Manganese), Mo (Molybdenum).
The Capture and Internment of Ms. Piggie
This is an interesting and funny story about how a pig ended up at our farm. We are not talking about one of those small pot-bellied pigs but a 450-lb Yorkshire sow! Of course, the pig was only about 20 lbs when we first saw her being chased by our two mustang horses in the pasture. Initially I wasn’t sure what they were chasing because you normally don’t see pigs running around the countryside! Later I discovered that the pig had escaped from our neighbor’s pen. He had recently purchased the pig as a pet for his daughter.
I contacted my neighbor and we devised a plan to capture the pig. Not being a pig expert, the plan seemed reasonable to me. Let the horses drive the pig from the pasture. My neighbor would then use his fishing net to throw over the pig. After several futile attempts, a pair of broken glasses, and a bruised knee (not to mention our bruised egos), the pig ran off into the woods. During the next two weeks we kept hearing reports of a pig traveling up and down the road in front of our house. In one case, someone mentioned that they had almost hit a pig while driving! Apparently the pig would use the road to find shelter in our woods in the evening and then reappear in the morning to do what pigs do best–pig rooting! Unfortunately for the pig, she was rooting our neighbors’ lawns. We figured it would not be long before she would end up on a spit over one of my neighbors’ evening campfires. Besides, a pig this smart had to be saved!
We built a small fenced enclosure and lured the pig inside during one of her daily excursions. We have had her ever since. Over time, we have grown to admire Ms. Piggie for her resourcefulness and intelligence. She is very gentle and even puts up with those crazy geese who regularly invade her house.
Pigs will eat most of your kitchen scraps and leftovers. She is particularly fond of pasta and goat milk, which we mix with a special blend of corn, soya meal and molasses. For parasite control, we occasionally add Safe-Guard (Panacur) dewormer (controls both roundworms and whipworms) in pellet form to her food (Kent Feeds). Containment and housing are sometimes a challenge. We have found by trial and error that the best option for housing is a 6×5-foot culvert secured with two metal T-posts on each side. The pig will build her own nest within the culvert. Just toss a few flakes of hay or straw into her pen and she will carry them (by mouth) into the culvert. You can use pig or goat panel for fencing; however, she has never tried to escape. I suppose she knows what awaits her on the outside.
After Ms. Piggie did so well on her own for several weeks, we began to wonder if domestic pigs bred for meat production could survive in this area as feral animals. Pigs are very adaptable and, as omnivores, could easily find food in the pastures and forests. As some of you might know, feral pigs are relatively common in the U.S., e.g., southern U.S., California and New England. However, these are invasive species, the result of crossbreeding between domestic pigs brought by the Spaniards to the Americas in the 1400 and 1500s, and wild boars introduced from Eurasia. They are smaller in size (100-200 lbs) than their domestic counterparts and more aggressive in nature.
Here are some pictures of Ms. Piggie as a piglet and later as a full grown pig (properly called a hog). I will add a few more images to the gallery when it’s a bit warmer.
- With Gus, soon after coming to farm
- With Silver, our miniature jack
- Several months after coming to farm
- With Gus, at 2 years of age
Tadpole farm in my horses’ stock tank!
Several weeks ago I noticed that our horses’ stock tank had been inhabited by tadpoles! If you are not familiar with stock tanks they are large (110-gallon capacity), oval-shaped galvanized water tanks designed for livestock. Apparently tree frogs had climbed into the tank and laid their eggs. It’s been very dry here this summer and most of Indiana has been classified as a drought area. Our pond has long since disappeared and there are very few places, if any, available for breeding. I guess the stock tank seemed like a nice place to propagate the species! After all, it is filled with non-chlorinated water from our well and there is plenty of room for a few dozen tadpoles.
Given the importance of frogs in the environment and the dire plight of amphibians worldwide, we wanted to make every attempt to ensure the survival of the tadpoles. Frogs can coexist with horses so we decided to let the tadpoles mature in the tank and climb out when they were ready. We refresh the tank each day to make sure that the temperature is tolerable and the water is oxygenated. When the horses drink they deposit hay and plant matter in the water which attracts insects, a food source for the tadpoles.
I thought it would be fun to document the development of the tadpoles with photos. This is sort of like the elementary school science project on the life cycle of frogs (It’s never too late to learn!). The tadpoles were too small to photograph soon after hatching so I waited until they began to develop their hind legs. At this stage the gills have been replaced by lungs. Eventually the front legs (forearms) will appear, the tail will shorten and the frogs will climb out of the tank to undergo a metamorphosis which will enable them to live on land. The first series of photos shows the transition from two to four legs which occurred at about 4-6 weeks (?) of age. If you examine the second photo in this series (tadpole dorsal) you will see two “bumps” located behind the eyes. These “bumps” mark the site of emergence of the front legs, elbow first.
Let’s all hope that the tadpoles reach maturity and we hear their wonderful song next spring!
Update
Shortly after the tadpoles developed four legs they disappeared from the stock tank. Unfortunately, we did not see the migration. We would like to think that they successfully made the transition to land and are thriving somewhere on our farm.
A Final note
If you are concerned about the fate of frogs and want to be informed, you need to read the Australian article by Dr. Sara Broomhall on “Frogs in an Effluent Society”. There is also an interesting article published recently by Raffel et al. (2012) on the possible effect of climate change on the threat of Chytridiomycosis, the parasitic fungus that is killing frogs worldwide.
Blood-Sucking Creatures in Your Goat’s Stomach!
I’m back with everybody’s favorite topic – worms, of course! I would put away that food you are munching on until you have finished reading this blog!
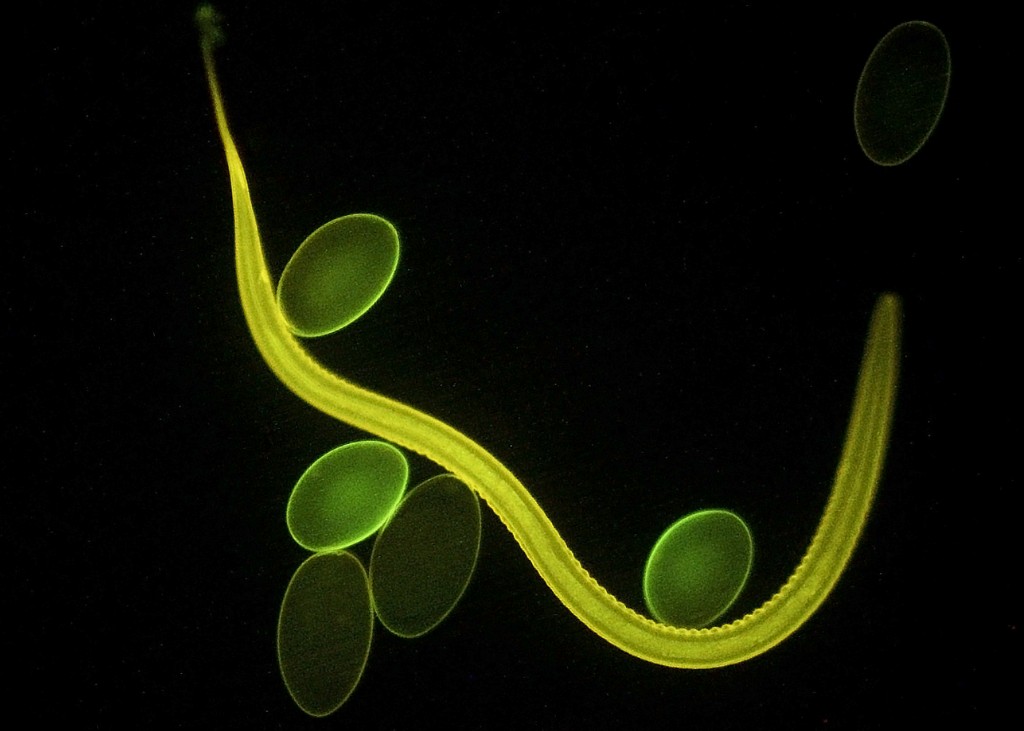
I want to share with you results from a simple comparative study that I carried out on common goat dewormers. It’s very unscientific but I thought it might be useful to some goat owners. As I mentioned in my previous goat blog, worms (parasites) are the number one problem affecting meat and dairy production worldwide, particularly in the Southern Hemisphere and Third World countries. It’s a major problem because goat worms are becoming resistant to all major groups of chemical dewormers (Kaplan 2013) The one parasite that’s the most serious threat is a guy called Haemonchus contortus, also known as the barber pole worm, or the blood worm. It makes a living sucking blood from the lining of your goat’s stomach and can cause severe anemia and, ultimately death, if not controlled.
Here’s an image of the culprit and some of its eggs captured with a fluorescence microscope. Ultraviolet (UV) light is causing the worm and eggs to “fluoresce”, or emit light in the visible portion of the spectrum. To give you an idea of scale, the long dimension of the eggs is about 100 micrometers, or 0.1 mm. This is a very ingenious application of the property of fluorescence to an important veterinary problem. I am sure that many of you have seen fluorescent mineral displays in geology departments and museums. It’s the same principle. Unlike some minerals, however, Haemonchus eggs are not naturally fluorescent. Therefore, the key is to find a chemical substance that (1) binds only to Haemonchus eggs in the fecal sample, and (2) fluoresces in UV light. In this case, the eggs are coated or “stained” with a plant protein (lectin) called peanut agglutinin. You can read a press release about the development and application of this technique at http://www.vet.uga.edu/pr/sheep-parasite.php.
Haemonchus contortus with several of its eggs as viewed with a fluorescence microscope. Photo credit: © 2009 The University of Georgia College of Veterinary Medicine.
Haemonchus is an example of a strongyle worm (small roundworm) found primarily in hotter, more humid climates. Unfortunately, its eggs cannot be distinguished from those of other species in this group based on size, or morphology (shape). The fluorescent staining method is a breakthrough in goat and sheep parasitology because it will now be possible to rapidly and inexpensively detect the presence of Haemonchus eggs and take the necessary steps to control this pathogenic organism (one worm can lay up to 10,000 eggs per day!).
I have set up my own small parasitology lab at our farm. It’s easy to do, relatively inexpensive and avoids the high cost of fecal tests, currently about $50.00 per test (quantitative analysis) at veterinary clinics in our area. I also determine my own egg counts for my mustang horses. It’s easy to learn to identify the most common parasites. For example, web sites such as the Maryland Small Ruminant Page, provide a number of general parasitology links with images and tutorials.
This is a photo of my lab for determining fecal egg counts using the McMaster technique. Other than a microscope, you will need a standardized fecal solution (in this case, sodium nitrate, or Fecasol, Specific Gravity =1.20) for floating the eggs, McMaster slides, gram scale (optional), an assortment of glassware, a strainer and funnel, and some exam gloves. I use the Modified McMaster Technique described by Ray Kaplan DVM, PhD and James Miller DVM, PhD in the article Modified McMaster Egg Counting for Quantitation of Nematode Eggs. I changed their procedure slightly by including a step to filter out the solid fraction in the fecal solution. This makes it easier to extract the liquid fraction and load the McMaster slide. When interpreting the results of the McMaster technique, it is important to remember that egg counts might not reflect the actual number of worms present. Refer to The RVC/FAO Guide to Veterinary Diagnostic Parasitology for a list of the factors that can affect egg counts. This web site is also a good source for other information about fecal analyses of small ruminants.
The objective of my experiments was to determine the dosage of chemical dewormers necessary to reduce the population of internal parasites by at least 90-95% after 2 weeks. This is referred to as the fecal egg count reduction test (FECRT) in the scientific literature. There are number of different types of parasites in goats but only those eggs belonging to the strongyle group were counted. These are the most common eggs observed in fecal analyses of goats.
The current recommendation by parasitologists is to use one dewormer until FECRT < 95%, indicating significant parasite resistance (S. Mobini, DVM, Fort Valley State University, pers. comm.). In practice, the actual FECRT% considered acceptable in parasite control programs will depend on a number of factors, including the availability of dewormers with higher efficacy (If a drug has high efficacy, 0% of worms will survive the dewormer). However, as FECRT results approach 80%, parasite resistance and associated health effects will begin to increase more rapidly. For example, in sheep FECRT < 80% has a negative effect on lamb growth rate.
Included in this study were Rumatel® (Morantel Tartrate), Panacur®/Safeguard® horse paste, Moxidectin horse paste (Quest® Gel) and liquid (injectable CYDECTIN®), Ivermectin (horse paste) and Dectomax® (injectable). For the time being, I did not consider Levamisole (Levasole, Tramisol and Prohibit® Drench), or some of the other benzimidazoles, e.g., Valbazen, which is unsafe for pregnant animals in first trimester. Because of the small number of animals in my herd (normally four), I have not used the oral drenches, or suspensions, recommended for goats, which are more bioavailable than horse pastes. Oral drenches, such as IVOMEC® sheep drench (goat dosage = 6 ml per 25 lbs body weight), are usually sold in 1-liter (1000 ml) containers with a 1-year expiration date.
I normally don’t deworm until the level of anemia is equal to, or greater than, “3” based on the FAMACHA system (“5” being severely anemic). The FAMACHA test compares the color of the lower eyelid to a color chart to determine the level of anemia from Haemonchus infection. If you are not familiar with FAMACHA, or parasite control in general, you can read about it at the web site for the American Consortium for Small Ruminant Parasite Control (ACSRPC).
Summary of results from comparative study using McMaster egg counting technique
Rumatel® (Morantel Tartrate): Only dewormer approved by FDA as an additive to goat feed. Advantage is that there is no withdrawal period for milk. Recommended dosage on bag is 2 lbs. In fact, it takes 2 lbs daily for two successive days to achieve FECRT > 95% after 2 weeks. Good alternative if you are already feeding processed food or milking your goats. I recently switched to Goat Care-2X medicated pellets (generic Rumatel® 88). Same stuff but more concentrated so one treatment (4 oz per 50 lbs body weight = 88 gm Morantel Tartrate per 100 lbs) will produce similar results for FECRT.
Panacur® (horse paste): One dose at 2-3 X body weight is ineffective (well known from goat literature). Three successive daily doses at 2-3 X body weight will reduce FECs by > 90% after 2 weeks (for any horse owners out there, this is the Panacur Powerpack for goats!). Panacur® and Safeguard® are the same drugs sold under a different label (Fenbendazole). The recommended goat dewormer is Safeguard®/Panacur® suspension (see ACSRPC table of anthelmintic dosage and withdrawal times below).
Moxidectin (Quest® Gel horse paste) versus liquid (injectable CYDECTIN®): FECRT for both dewormers was > 90% after 2 weeks. Dosage for paste is 2 X body weight, that for liquid only 1 X, as recommended by ACSRPC. Downside to using the injectable liquid is cost for a small herd — about $110.00 for 200 ml. Philosophy behind using the liquid is that less is required (it has a longer residence time, or persistence, in the animal’s system) so parasite drug resistance will develop more slowly. As it turns out, the injectable form of Moxidectin (CYDECTIN®) is no longer recommended for use by the ACSRPC because of its greater withdrawal times (120-130 days) for consumption of meat. In addition, a recent study indicates that Moxidectin has a higher efficacy when administered orally in cattle (CYDECTIN® Drench) rather than injected subcutaneously, or applied topically (pour-on). Oral use of pour-on CYDECTIN® is not recommended for goats.
Ivermectin (horse paste) and Dectomax® (injectable) (avermectin group): Not effective in significantly reducing FECs for my small herd (FECRT << 80%). Neither the Ivermectin (IVOMEC®) sheep drench, or injectable ivermectin (IVOMEC®), administered orally or subcutaneously, were used in this limited study. Both routes of injectable ivermectin are considered (extremely) extralabel because the IVOMEC sheep drench is available. In addition, the formulation (solution of chemical substances plus active drug) for the oral drench is more bioavailable than ivermectin injectable given orally (Patty Scharko, DVM, Clemson University, pers. comm.). For those of you with lactating dairy goats, note that ivermectin injectable has a 40-day withdrawal time for milk (FARAD Digest 2000).
For reference, anthelmintic dosage and withdrawal times for goats are given on the ACSRPC web site. Note that Rumatel® 88 listed in the footnote to ACSRPC table has 0 days milk withdrawal time for 88 gm/100 lbs body weight dose (refer to withdrawal charts in Compendium of Veterinary Products 2014).
A few concluding remarks about the drug class of macrocyclic lactones (MLs), which includes two closely related chemical groups – the avermectins and milbemycins. Ivermectin and Doramectin (“Dectomax®”) are avermectins whereas Moxidectin is a member of the milbemycin group. Ivermectin and Moxidectin have a longer persistence in the body, which means that these dewormers can persist in lethal concentrations for an extended period of time. Relative to Ivermectin, Moxidectin provides a greater persistent period and a greater efficacy against resistant worms.
Keep in mind that Ivermectin has been used extensively in goats since the 1980s so one might expect a greater parasite resistance to this drug. Moxidectin is a more potent drug and the last of the MLs to be introduced. Therefore, parasite drug resistance should develop more slowly. Unfortunately, Moxidectin is no longer effective in controlling parasites in many sheep and goat farms in the eastern and southern U.S. (Kaplan 2013). This trend will continue if we don’t make judicious use of dewormers, and employ effective pasture management practices that disrupt the life cycle of parasites.
So far we have only talked about chemical dewormers. There are other alternatives such as herbal remedies and pasture plants but how effective are they in controlling internal parasites? More on this topic at the ACSRPC web site.
At present, we are trialing the herbal product Verm-X (certified organic, available from ebay) and pasturing our goats in areas where the tannin-rich plant Sericea lespedeza is particularly abundant. For a nutrient profile (major and trace minerals) of Sericea lespedeza (a legume) link to my blog article on Pasture Weeds as Mineral Sources for Goats.
As a disclaimer, I need to mention that I am not a veterinarian and cannot provide medical advice for your goats. All the information presented here is based on work with my dairy goats, the ACSRPC web site and what I have read in the scientific literature. Updated July 17, 2014
Milking My Goat Fanny
I have always loved goat milk and cheese. When I was finally able to raise my own goats, I began scouring the literature for information on milking goats. As you can imagine, there isn’t one accepted way to produce good milk and cheese. Therefore, I decided to use this blog to say a few words about my own experience milking my Alpine goat, Fanny, for about three years.
Fanny is more than four years removed from kidding after her first freshening. At the moment, she is still producing about one-half gallon of milk per day. I never pasteurize the milk, or sterilize my milking container, which is a half-moon stainless steel bucket. I like the half-moon design because it keeps particulates out of the milk. After each milking, the milk is filtered into glass jars with a stainless steel funnel, precooled to ~45 F within 1 hr (jar placed in ice bath in freezer at 0 F) and then refrigerated. It usually lasts about two weeks at 37 F.
Although some goat people milk in the aisles of their barn with no restraint, I decided to build a wooden platform with a stanchion. It is housed in a small room with a wooden floor in one of our barns. Before I begin milking, I thoroughly wash the udder and my hands with Wipe Out Diary Wipes. I like using the wipes because you can eliminate the step of drying the udder. I have never shaved the udder, or used any other products to clean the udder. I direct the first few streams of milk into a strip cup to check for any unusual texture, which might indicate mastitis. Every two weeks or so, I also test for mastitis either with the California Mastitis Kit, or with a simple color indicator. When I am finished milking, I spray the opening in the teats with Fight Bac, a chlorhexidine gluconate antibacterial (common scrub in hospitals). The spray also contains glycerine, which is added to help control any chapping, or irritation of the teats. I prefer the spray over a teat dip, which easily becomes contaminated with hair and other stuff. Since I started milking I have never had any problems with contaminated milk, or mastitis.
I have to mention something about feeding Fanny during milking, and her unusual eating habits. I feed her Purina’s Noble Goat Dairy Parlor (16% protein in pellet form). She just gobbles her food, which is the reason I switched from sweet feed to pellets. In her case, you also have to add a little water with each serving to prevent choke. I add the food to her feeding tray in increments of about two handfuls. When she finishes each batch she starts this dancing routine and looks back at me as if to say, “Give me more”! If you try to milk without providing the food you run the risk of her kicking the milking bucket and creating a big mess. I have read about ways to restrain her back legs but it’s just not worth it. At the moment, I am only milking one goat which takes me about 15 minutes from start to finish.
So there you have it! Milking a goat is fun and you can easily produce high quality milk and cheese without a big time investment. Goat milk also makes a wonderful yogurt. I was finally able to find a simple yogurt maker (Euro Cuisine) that holds seven 6-ounce glass jars.
In my next goat blog, I will talk about my experiences with parasite control, the number one problem facing meat and diary production worldwide. Stay tuned!
The champignon
It was great year for pasture mushrooms (Agaricus campestris). They thrive on all that horse manure in our fields!
When I collect mushrooms it brings me back to my childhood and a special story involving my dad and my French grandfather. My mom was from Lyon in the Provence region of southern France. My dad and her were married in France at the end of World War II and eventually settled in my dad’s hometown in southern Illinois. My French grandparents would occasionally come to visit us during the summer months. My grandfather was a very particular man and a connoisseur of food and wine. Like most Europeans, he was also an expert in identifying edible mushrooms. It so happened that one evening he came home with a bag of pasture mushrooms that he had collected around our farm. My mom prepared the mushrooms precisely according to his instructions. I remember that he even chose the wine (French, of course!) for the meal. The mushrooms were delicious! However, we all noticed that my dad was not eating the mushrooms but, instead, was intently watching my grandfather as he consumed one mushroom after another. We finally discovered that my dad was determined not to eat a single mushroom until he was certain that they would not make my grandfather sick! It took a long time before my grandfather would forgive him for not trusting a French mushroom expert. A few years later my dad developed his own interest in mushrooms and we would go collecting together. So when I collect “champignon”, as my mother referred to them, I always remember my French heritage and the time I had with my dad as a kid in Illinois.
Early Morning Visitors
My first blog has to do with this very interesting picture, which I took looking out the window behind my computer. It was about 3:00 am and I just happened to look up from my screen. These little guys are peepers – the small tree frogs with sticky toepads who sing those wonderful choruses after a spring /summer rain. My lamp had apparently attracted some small tasty moths. What struck me was the almost perfect symmetrical arrangement of the frogs at the corners of a square. I was trained in school as a crystallographer, one of those guys who studies symmetry in crystals and minerals. I guess that’s why I always look for symmetry in nature. Perhaps the 4-fold symmetry was the most efficient way to ensure that each frog had an equal share of the morning meal! Since the weather has turned cooler here I don’t see them anymore. I miss watching them.